Introduction
Type 2 diabetes (T2D) is a global epidemic with changing demographics that poses a huge challenge for the health and economy of all nations and ethnicities. Ethnicity is defined in terms of a culture of people in a given geographic region, including their language, heritage, religion and customs.1 South Asians constitute about 1.6 billion people from the countries of the Indian subcontinent, this includes Afghanistan, Bangladesh, Bhutan, India, Maldives, Pakistan, Nepal and Sri Lanka.2 Over 37 million ethnic South Asian diaspora live across every continent.3
Prevalence of diabetes in South Asians
According to the International Diabetes Federation, diabetes affects at least 415 million people worldwide, and that number is expected to reach 642 million by the year 2040, with two-thirds of all diabetes cases occurring in low- to middle-income countries.4 Diabetes was once thought to be a ‘disease of the West’ and a ‘disease of affluence’ but it is now increasingly common among the poor and in certain ethnic groups. The prevalence of diabetes in India during the period 1971–2014, from 39 studies, showed an alarming increase in incidence of diabetes both in urban – 1.2% (1972) to 16.4% (2014) – and in rural India – 1.3% (1979) to 19.8% (2014).5 The prevalence of diabetes is mirrored in Pakistan and Sri Lanka, with rises seen in both urban and rural areas.6,7
According to the UK 2011 Census, Asian/Asian British account for 7.5% of the UK population (England and Wales) of which, 2.5% were Asian/Asian British Indian, 2.0% Asian/Asian British Pakistani, 0.8% were Asian/Asian British Bangladeshi.8 In 2010, the South Asian Health Foundation (SAHF) estimated that there were 388,000 South Asian people with T2D in the UK, equating to 15% of the population of people with diabetes, followed by Afro-Caribbean (11%) and white, mixed or other (7%).9
Health Survey of England (1999) and Diabetes UK (2004) reported that the risk of T2D is increased up to six-fold in UK South Asians, with the highest standardised risk ratio (SRR) of 2.92 among Indians, <5.5 in Pakistanis, and <5.7 in Bangladeshis, as compared with the general population (1.0).10,11
A systematic review and meta-analysis by Meeks K et al.12 quantified the variation in T2D risk among ethnic minority populations of different geographical origin compared with the host European populations. Compared with the host populations, South Asian origin populations had the highest odds for T2D (3.7, 95% confidence interval [CI] 2.7 to 5.1), followed by Middle Eastern and North African (MENA) (2.7, 95%CI 1.8 to 3.9), Sub-Saharan African (SSA) (2.6, 95%CI 2.0 to 3.5), Western Pacific (WP) (2.3, 95%CI 1.2 to 4.1), and lastly South and Central American (SCA) (1.3, 95%CI 1.1 to 1.6). Odds ratios were, in all ethnic minority populations, higher for women than for men, except for SCA. Among South Asian subgroups, compared with Europeans, Bangladeshi had the highest odds ratio of 6.2 (95%CI 3.9 to 9.8), followed by Pakistani (5.4, 95%CI 3.2 to 9.3) and Indians (4.1, 95%CI 3.0 to 5.7). The risk of T2D among ethnic minority groups living in Europe compared with Europeans varied by geographical origin of the group: three to five times higher among South Asians; two to four times higher among MENA; and two to three times higher among SSA origin.12
A cross-sectional analysis comparing age-specific prevalence of pre-diabetes, T2D, and the associated risk factors between Asian Indians living in the US and India, the CARRS (India) and MASALA (US) studies have also shown that age-adjusted diabetes prevalence was higher in India (38%, 95%CI 36 to 40) than in the US (24%, 95%CI 21 to 27). Age-adjusted pre-diabetes prevalence was lower in India (24%, 95%CI 22 to 26) than in the US (33%, 95%CI 30 to 36). After adjusting for age, sex, waist circumference, and systolic blood pressure; living in the US was associated with an increased odds for pre-diabetes (odds ratio 1.2, 95%CI 0.9 to 1.5) and a decreased odds for diabetes (odds ratio 0.5, 95%CI 0.4 to 0.6) in South Asians relative to their indigenous counterparts. This study indicates shift in the association between migration and diabetes risk and highlights the growing burden of disease in urban India.13
Diabetes as a risk factor for adverse outcomes
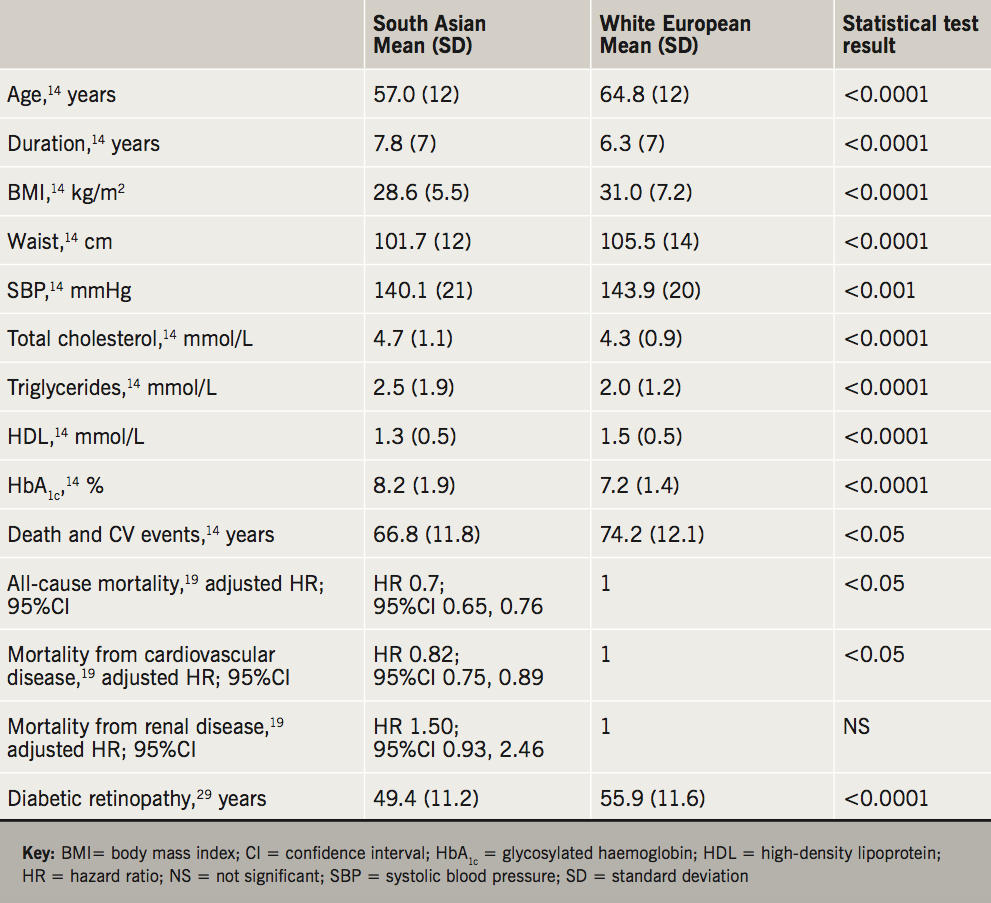
The risk profiles in South Asian and white European (WE) patients from UKADS (United Kingdom Asian Diabetes Study) showed significant findings in South Asians who tend to have early onset of diabetes (57.0 vs. 64.8 years), for longer duration (7.8 vs. 6.3 years), with individuals of lower body mass index (BMI) (28.6 vs. 31.0 kg/m2), lower waist circumference (101.7 vs. 105.5 cm) lower systolic blood pressure (SBP) (140.1 vs. 143.9 mmHg), lower high-density lipoprotein (HDL) (1.3 vs. 1.5 mmol/L) but had relatively higher total cholesterol (4.7 vs. 4.3 mmol/L), higher triglycerides (2.5 vs. 2.0 mmol/L) and higher glycosylated haemoglobin (HbA1c) (8.2% vs. 7.2%) when compared with WEs.14 Data from UKADS also showed ethnic differences in HbA1c in relation to duration, with HbA1c gradually rising from 7.6% to 9.1% in South Asians while WE showed an increase from 6.8% to 7.4% over 20 years.15
People with T2D have a two- to fourfold increased risk of cardiovascular disease compared with people without diabetes. Death and cardiovascular events were almost 50% higher in South Asians than in WE with T2D.16-18 Studies using THIN (The Health Improvement Network) database also showed that the risk of developing T2D is highest in South Asians at a lower BMI when compared with either WE or Afro-Caribbeans. It also showed, irrespective of levels of BMI, diabetes is associated with significantly greater risk of major adverse cardiovascular events (MACE) and that South Asians show greater susceptibility to MACE and chronic kidney disease (CKD) even at lower BMI levels. Overall, the risk of developing MACE in patients with T2D, compared with non-diabetic controls was significantly higher for South Asians (95%CI of incidence rate ratio [IRR] 1.56 to 2.22) compared to WEs (95%CI of IRR 1.29 to 1.38) at younger age.19,20
In South Asians, the key conventional coronary heart disease (CHD) risk factors include smoking and total cholesterol, and the adverse risk factors include insulin resistance, dyslipidaemia and hyperglycaemia. In the South Asian group, diabetes increased the mortality risk nearly threefold compared with South Asians without diabetes at baseline. In Europeans, the excess mortality associated with diabetes was only 1.5-fold and there is 60% greater CHD death in South Asians than in Europeans independent of traditional risk factors.21 Although the reasons for excess CHD mortality risk in South Asians are not entirely clear, the INTERHEART (Effect of Potentially Modifiable Risk Factors Associated with Myocardial Infarction) study found higher levels of conventional risk factors present at a younger age to be the likely explanation for occurrence of myocardial infarction at an earlier age in the South Asian population.18 Coronary risk factors in UK South Asian men and their non-migrant siblings in Punjabi India also showed significant increase with high BMI (26.8 vs. 22.9 kg/m2), high SBP (146 vs. 132 mmHg), high diastolic blood pressure (DBP) (93 vs. 87 mmHg), high total cholesterol (6.5 vs. 4.9 mmol/L) and fasting glucose (5.7 vs. 4.5 mmol/L).22 Similar impact of migration on CHD risk factors in Gujarati Asians were also seen in a 242 population of migrant Gujarati Indians at Sandwell and 294 from Navsari (India). Migrants showed increased CHD risk with higher cholesterol (5.36 vs. 4.82 mmol/L), triglycerides (1.22 vs. 0.91 mmol/L), BMI (25.9 vs. 21.0 kg/m2), waist-to-hip ratio (0.92 vs. 0.87), hypertension (46.9% vs. 23.7%), physical activity (2,000 vs. 1,700 kcal/day) and energy intake (2,000 vs. 1,350 kcal/day) and body fat percentage (39% vs. 31%).23 Asymptomatic left ventricular hypertrophy, diastolic dysfunction and heart failure with normal ejection fraction are common in people with diabetes and impaired glucose regulation (IGR).24
However, recent data from Wright et al. suggest CHD risk levels may be declining over time. In white men and women, the effect of diabetes on life-expectancy loss estimates were greater than in South Asian and Black individuals.25 As summarised in table 1, the presence of diabetes in older South Asians was associated with modest protection from all-cause mortality. This could partly be due to treatment of South Asian patients with lifestyle intervention and medication at a young age, often before the development of macrovascular disease. Such earlier treatment may have slowed the progression of atherosclerosis and lowered the risk of cardiovascular death compared with those without diabetes. There is a paucity of evidence regarding heart failure, the prevalence, prognosis and outcome in different ethnic groups is not well documented. This latter study requires verification from other contemporary cohorts, but if findings are verified, this would be encouraging news.
Like CHD, stroke is also more common in the South Asian population, presents at a younger age, and has a 40% higher mortality compared with a western population.25,26 Older age and the presence of atrial fibrillation are key factors related to the high stroke-related mortality in Europeans.26 Data from a 17-year mortality follow-up of the Southall and Brent cohort showed diabetes as a strong predictor of stroke mortality in South Asians compared with Europeans,27 and similar findings were seen in studies from the USA and India.28,29
The relative risk (RR) of diabetic renal disease among South Asians is higher (RR 13.6, 95%CI 6.1 to 30.6) when compared with non-diabetic South Asians (RR 3.6, 95%CI 2.6 to 4.4). At a much younger age, South Asian T2D patients develop more nephropathy and have progressive renal failure in comparison with European diabetic patients. At least a threefold increased risk of end-stage renal disease (ESRD) is now confirmed in South Asians and Afro-Caribbeans (UK Renal Registry and US Renal Data System).30
The risk of diabetic retinopathy (DR) is also higher among South Asians when compared with WE. Diabetes occurs at an earlier age among South Asians (49.4 vs. 55.9 years) with DR also occurring early (55.2 vs. 65.9 years) and there is higher incidence of DR with a significantly lower duration of diabetes (7.8 vs. 9.1 years).31
Contributing factors towards poor diabetes control
What are the reasons behind poor control of diabetes among South Asian communities? Comparatively low socio-economic position, cultural barriers, language barriers, fatalistic attitude and cultural ‘taboos’ along with genetic, epi-genetic and lifestyle factors are cited as possible explanations. Among the lifestyle factors, overweight and obesity are important contributors to the global epidemic of diabetes (figure 1). The definitions of obesity in South Asians are at a lower BMI for the diabetes risk.32 High levels of smoking, along with high-fat diets and low levels of exercise, are common among some, but not all, South Asian communities.33-35 In South Asians, increased risk of diabetes starts at a much lower BMI than for WE people; for any given BMI, South Asians have a tendency toward higher abdominal fat mass in deep abdominal subcutaneous and visceral depots; there is emerging evidence for greater liver fat too, although further studies are needed. South Asians have a low proportion of lean muscle mass, which might contribute to their higher insulin resistance and diabetes risk. In addition, even a modest amount of weight gain during adulthood substantially increases the risk of diabetes in Asians.36 This ‘metabolically obese’ phenotype among normal-weight South Asian individuals may explain the increased predisposition for diabetes, despite a relatively low prevalence of obesity. Computer-based tomography has shown that South Asians have more visceral fat than Whites with the same waist circumference.37 It is hypothesised, but not proven, that the high prevalence of insulin resistance, and lifelong compensatory hyperinsulinaemia, drives the high rates of microalbuminuria and ischaemic heart disease.38 This ethnic susceptibility for insulin resistance could explain the high risk for T2D, coronary disease, microalbuminuria, and renal failure in South Asians.
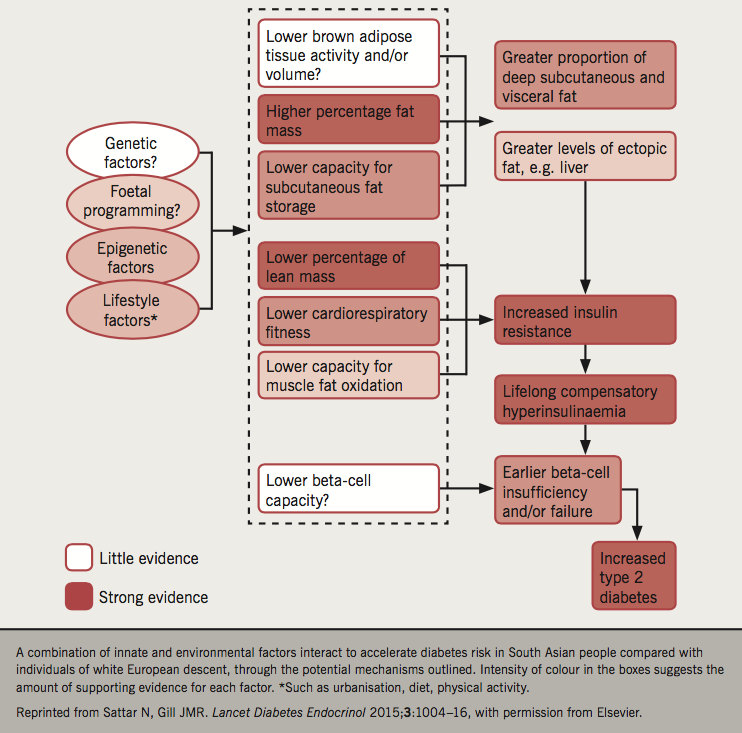
Is the increased T2D in the South Asian population associated with gene–environment interactions of known susceptibility genes? Genome-wide association study (GWAS) in individuals of South Asian ancestry does not provide a genetic explanation for the high risk of T2D in South Asians, but does provide insight into genetic mechanisms underlying T2D.39 At least seven different types of monogenic forms of diabetes (MODY) have been described, but studies involving South Asians are limited, and there is a considerable overlap of common T2D with MODY, making it difficult to estimate the true prevalence.40 Six novel risk loci for T2D have been identified and all had similar effects on disease risk in South Asians and WE.41 So far, only transcription factor 7 like 2 (TCF7L2) has been shown to have strong association with the risk of T2D in the South Asian population, but the exact role of this gene in the pathogenesis of T2D is unknown.42 GWAS has also identified genes associated with obesity, a major risk factor for T2D. Common variants of the fat-mass and obesity-associated gene (FTO) were shown to be associated with obesity in European populations while in South Asians, however, the presence of this polymorphism was associated with an increased risk of T2D independent of BMI.43 A meta-analysis has shown low birth weight to be associated with greater risk of T2D, with each kg increase associated with roughly a 25% decrease in diabetes risk. The Born in Bradford study showed a link between higher maternal glucose levels and greater foetal fat mass.44 The START (South Asian Birth Cohort) study also showed lower birth weight with higher measures of fat mass in South Asian newborns when compared with WE.45
Gene–environment–lifestyle exposure may underlie the progressive elevated risk for T2D development in South Asians. Genetic factors have not currently been clearly identified. There is some evidence emerging of epigenetics in the causation, but this needs to be proven to be causal by interventional trials. Fat distribution, lean body mass and brown fat have all been implicated. Lifestyle is perhaps the most important modifiable risk factor with strongest evidence, both in terms of prevalence and interventions in prevention.
The role of ethnicity in disease causation remains unclear. Currently, there are no ethnic-specific screening strategies, and ethnicity as a risk factor for diabetes and diabetes-related complications is not universally recognised, although it is now common in many risk scores as a risk modifier. Investigations into the causes that lead to this excess diabetes risk and better strategies to effectively manage these patients once diabetes occurs are clearly needed to reduce the morbidity and mortality associated with this condition.
Management of diabetes
In a meta-analysis published by Khunti et al. in 2015, the number of South Asians in randomised-controlled trials was very low.46 It was hard to postulate the advantages of one antidiabetes drug over others. However, recently published clinical trials (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients–Removing Excess Glucose [EMPA-REG OUTCOME] and Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results [LEADER]), with modest South Asian cohorts, do suggest a possible beneficial effect of sodium-glucose co-transporter 2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) analogues over other medications, although these are post-hoc analyses and results should be interpreted cautiously. The consensus statements encourage less use of medications causing weight gain and hypoglycaemia in these populations. These recommendations are based on a pragmatic approach rather than good evidence. As a way forward we need more clinical trials in South Asian cohorts, or to have certain proportions of them in large randomised-controlled trials, in order to have a better understanding of treatment algorithms in this group.
Key messages
- Prevalence of diabetes is high in people with South Asian heritage, along with an increased risk of complications (coronary heart disease, diabetic retinopathy and end-stage renal disease) occurring at younger age with lower duration of diabetes, although some recent evidence suggests cardiovascular disease death risks may have declined even more in South Asians, but this needs verification
- Diabetes occurs at a lower body mass index (BMI) in this group due to altered body composition, meaning South Asians should be encouraged to maintain lower BMIs lifelong and to keep as active as possible
- Younger patients with diabetes should be managed aggressively to reduce their risk of developing complications linked, in particular, to hyperglycaemia. Currently, limited evidence is available to devise an algorithm for these patients, hence, more research is needed
Conflict of interest
WH has received travel grants, research grant and consultancy fees from the following companies: Novo Nordisk, Eli Lilly, Sanofi, MSD, Jansen, Astra Zeneca and BI. RS: none declared
Other articles in this supplement include:
Introduction
1. The cardiovascular profile in diabetes
3. Drugs for diabetes: the cardiovascular evidence base
4. Optimising cardiovascular risk reduction in diabetes
References
1. Live Science. What is the difference between race & ethnicity? May 2012. Available at: http://www.livescience.com/33903-difference-race-ethnicity.html [accessed 26 September 2016].
2. South Asian Concern. Who are South Asians. Available at: http://southasianconcern.org/south-asian/ [accessed 26 September 2016].
3. NYU Shanghai Library. Asian/Pacific diaspora and migration. Available at: http://guides.nyu.edu/asiandiaspora [accessed 26 September 2016].
4. International Diabetes Federation. IDF Diabetes Atlas. 7th Edition. Brussels: IDF, 2015. Available from: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/13-diabetes-atlas-seventh-edition.html
5. Tandon N, Raizada N. The burden of diabetes in India [internet]. Diapedia 2014;1105045828 rev. no. 8. https://doi.org/10.14496/dia.1105045828.8
6. Hakeem R, Fawwad A. Diabetes in Pakistan: epidemiology, determinants and prevention. Journal of Diabetology 2010;3:4. Available from: http://www.journalofdiabetology.org/temp/JDiabetol133-4159812_113318.pdf
7. Katulanda P, Ranasinghe P, Jayawardena R, Sheriff R, Matthews D. Metabolic syndrome among Sri Lankan adults: prevalence, patterns and correlates. Diabetol Metab Syndr 2012;4:24. https://doi.org/10.1186/1758-5996-4-24
8. Office of National Statistics. Ethnicity and national identity in England and Wales 2011. London: ONS, December 2011. Available from: http://webarchive.nationalarchives.gov.uk/20160105160709/http://www.ons.gov.uk/ons/dcp171776_290558.pdf [accessed 26 September 2016].
9. Hanif W, Khunti K, Bellary S et al. Type 2 diabetes in the UK South Asian population. An update from the South Asian Health Foundation. Birmingham: South Asian Health Foundation, 2014. Available from: https://www.sahf.org.uk/resources/
10. Health and Social Survey Research Group. Health Survey for England. London: The Stationery Office, 2001.
11. Watkins PJ. Diabetes and its Management. Oxford: Blackwell Publishing, 2003. https://doi.org/10.1002/9780470760277
12. Meeks KAC, Freitas-Da-Silva D, Adeyemoet A et al. Disparities in type 2 diabetes prevalence among ethnic minority groups resident in Europe: a systematic review and meta-analysis. Intern Emerg Med 2016;11:327–40. https://doi.org/10.1007/s11739-015-1302-9
13. Gujral UP, Narayan KMV, Pradeepa RG et al. Comparing type 2 diabetes, prediabetes, and their associated risk factors in Asian Indians in India and in the U.S.: the CARRS and MASALA studies. Diab Care 2015;38:1312–18. https://doi.org/10.2337/dc15-0032
14. Bellary S, O’Hare JP, Raymond NT et al. Premature cardiovascular events and mortality in south Asians with type 2 diabetes in the United Kingdom Asian Diabetes Study – effect of ethnicity on risk. Curr Med Res Opin 2010;26:1873–9. https://doi.org/10.1185/03007995.2010.490468
15. Results from the United Kingdom Asian Diabetes Study analysed by the speaker http://www.tandfonline.com/doi/pdf/10.1185/03007995.2010.490468?needAccess=true [accessed 26 September 2016].
16. Wilkinson P, Sayer J, Laji K et al. Comparison of case fatality in south Asian and white patients after acute myocardial infarction: observational study. BMJ 1996;312:1330–3. https://doi.org/10.1136/bmj.312.7042.1330
17. Joshi P, Islam S, Pais P et al. Risk factors for early myocardial infarction in South Asians compared with individuals in other countries. JAMA 2007;297:286–94. https://doi.org/10.1001/jama.297.3.286
18. Forouhi NG, Sattar N, Tillin T, McKeigue PM, Chaturvedi N. Do known risk factors explain the higher coronary heart disease mortality in South Asian compared with European men? Prospective follow-up of the Southall and Brent studies, UK. Diabetologia 2006;49:2580–8. https://doi.org/10.1007/s00125-006-0393-2
19. Owusu Adjah ES, Bellary S, Hanif W, Patel K, Khunti K, Paul SK.Prevalence and incidence of complications at diagnosis of T2DM and during follow-up by BMI and ethnicity: a matched case-control analysis. Cardiovasc Diabetol 2018;17:70. https://doi.org/10.1186/s12933-018-0712-1
20. Paul SK, Owusu Adjah ES, Samanta M et al. Comparison of body mass index at diagnosis of diabetes in a multi-ethnic population: a case-control study with matched non-diabetic controls. Diabetes Obes Metab 2017;19:1014–23. https://doi.org/10.1111/dom.12915
21. Bhatnagar D, Anand IS, Durrington PN et al. Coronary risk factors in people from the Indian subcontinent living in West London and their siblings in India. Lancet 1995;345:405–09. https://doi.org/10.1016/S0140-6736(95)90398-4
22. Patela JV, Vyas A, Cruickshank JK et al. Impact of migration on coronary heart disease risk factors: comparison of Gujaratis in Britain and their contemporaries in villages of origin in India. Atherosclerosis 2006;185:297–306. https://doi.org/10.1016/j.atherosclerosis.2005.06.005
23. Dawson A, Morris AD, Struthers D. The epidemiology of left ventricular hypertrophy in type 2 diabetes mellitus. Diabetologia 2005;48:1971–9. https://doi.org/10.1007/s00125-005-1896-y
24. Lee CM, Huxley RR, Woodward M et al. Prevalence of diabetes mellitus and population attributable fractions for coronary heart disease and stroke mortality in the WHO South-East Asia and Western Pacific regions. Asia Pac J Clin Nutr 2007;16:187–92. Available from: http://apjcn.nhri.org.tw/server/APJCN/16/1/187.pdf
25. Wright AK, Kontopantelis E, Emsley R et al. Life expectancy and cause-specific mortality in type 2 diabetes: a population-based cohort study quantifying relationships in ethnic subgroups. Diabetes Care 2017;40:338–45. https://doi.org/10.2337/dc16-1616
26. Gunarathne A, Patel JV, Potluri R et al. Increased 5-year mortality in the migrant South Asian stroke patients with diabetes mellitus in the United Kingdom: the West Birmingham Stroke Project. Int J Clin Pract 2008;62:197–201. https://doi.org/10.1111/j.1742-1241.2007.01580.x
27. Southall and Brent revisited (SABRE) website. Available at: http://www.sabrestudy.org
28. Moussouttas M, Aguilar L, Fuentes K. Cerebrovascular disease among patients from the Indian subcontinent. Neurology 2006;67:894–6. https://doi.org/10.1212/01.wnl.0000233923.63869.8c
29. Kaul S, Sunitha P, Suvarna A. Subtypes of ischemic stroke in a metropolitan city of south India (one year data from a hospital based stroke registry). Neurol India 2002;50:S8–S14.
30. Burden AC, McNally PG, Feehally J, Walls J. Increased incidence of end-stage renal failure secondary to diabetes mellitus in Asian ethnic groups in the United Kingdom. Diabet Med 1992;9:641–5. https://doi.org/10.1111/j.1464-5491.1992.tb01860.x
31. Raymond NT, Varadhan L, Reynold DR et al. Higher prevalence of retinopathy in diabetic patients of South Asian ethnicity compared with white Europeans in the community. Diabetes Care 2009;32:410–15. https://doi.org/10.2337/dc08-1422
32. Yates T, Davies MJ, Gray LJ et al. Levels of physical activity and relationship with markers of diabetes and cardiovascular disease risk in 5474 white European and South Asian adults screened for type 2 diabetes. Prev Med 2010;51:290–4. https://doi.org/10.1016/j.ypmed.2010.06.011
33. NHS Digital. Health Survey for England – 2004: health of ethnic minorities. Headline results. Available at: https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/health-survey-for-england-2004-health-of-ethnic-minorities-headline-results
34. Yoon KH, Lee JH, Kim JW et al. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006;368:1681–8. https://doi.org/10.1016/S0140-6736(06)69703-1
35. Misra A, Vikram NK. Insulin resistance syndrome (metabolic syndrome) and obesity in Asian Indians: evidence and implications. Nutrition 2004;20:482–91. https://doi.org/10.1016/j.nut.2004.01.020
36. Popkin BM. Nutrition in transition: the changing global nutrition challenge. Asia Pac J Clin Nutr 2001;10(suppl):S13–S18. https://doi.org/10.1046/j.1440-6047.2001.0100s1S13.x
37. Sattar N, Gill JMR. Type 2 diabetes in migrant south Asians: mechanisms, mitigation, and management. Lancet Diabetes Endocrinol 2015;3:1004–16. https://doi.org/10.1016/S2213-8587(15)00326-5
38. Hu FB, Manson JE, Stampfer MJ et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345:790–7. https://doi.org/10.1056/NEJMoa010492
39. Frayling TM, McCarthy MI. Genetic studies of diabetes following the advent of the genome-wide association study: where do we go from here? Diabetologia 2007;50:2229–33. https://doi.org/10.1007/s00125-007-0825-7
40. Malecki MT. Genetics of type 2 diabetes mellitus. Diabetes Res Clin Pract 2005;68(suppl 1):S10–S21. https://doi.org/10.1016/j.diabres.2005.03.003
41. Lawlor DA, West J, Fairley L et al. Pregnancy glycaemia and cord-blood levels of insulin and leptin in Pakistani and white British mother-offspring pairs: findings from a prospective pregnancy cohort. Diabetologia 2014;12:2492–500. https://doi.org/10.1007/s00125-014-3386-6
42. Zeggini E, McCarthy MI. TCF7L2: the biggest story in diabetes genetics since HLA? Diabetologia 2007;50:1–4. https://doi.org/10.1007/s00125-006-0507-x
43. Radha V, Mohan V. Genetic predisposition to type 2 diabetes among Asian Indians. Indian J Med Res 2007;125:259–74.
44. West J, Santorelli G, Whincup PH et al. Association of maternal exposures with adiposity at age 4/5 years in white British and Pakistani children: findings from the Born in Bradford study. Diabetologia 2018;61:242–52. https://doi.org/10.1007/s00125-017-4457-2
45. Annand SS, Vasudevan A, Gupta M et al. Rationale and design of South Asian Birth Cohort (START): a Canada-India collaborative study. BMC Public Health 2013;13:79. https://doi.org/10.1186/1471-2458-13-79
46. Khunti K, Bellary S, Karamat MA et al. Representation of people of South Asian origin in cardiovascular outcome trials of glucose-lowering therapies in type 2 diabetes. Diabet Med 2017;34:64–8. https://doi.org/10.1111/dme.13103


