Over 50,000 cardiac implantable electronic device procedures are undertaken annually in the UK. Despite prophylactic measures, device infection still occurs. Anaphylaxis following teicoplanin is extremely rare with evidence limited to case reports and one case series. We present two fatal cases of anaphylaxis following teicoplanin administration. Both cases meet the World Allergy Organisation definition of anaphylaxis. These cases highlight the importance of anaphylaxis to teicoplanin as a procedural complication. Despite prompt treatment, this reaction was fatal. Operators should be aware of this risk in an era of increasing procedures and rising incidence of anaphylaxis.
Introduction
Each year over 50,000 cardiac implantable electronic device (CIED) procedures are undertaken in the UK.1 Despite prophylactic measures, device infection, one of the more severe complications, still occurs. This complication is associated with hospital admissions, increased mortality and cost.2-4 The majority of infections are caused by Staphylococcus spp. (60–80%) with the remainder predominantly caused by other Gram-positive cocci, Gram-negative bacilli and candida spp.5,6 Retrospective analysis of over 200,000 implantable cardioverter defibrillator procedures showed increased infection risk to be associated with generator change, device upgrade, re-intervention, previous valvular surgery, renal failure, chronic lung disease, cerebrovascular disease and warfarin therapy.7 This analysis reported infection rates of 1.4%, 1.5% and 1.8% for single, dual and cardiac resynchronisation therapy devices, respectively.7 Similar figures have been reported across the range of CIEDs (0.5–2.2%).8
The use of peri-procedural antibiotics has been shown to significantly reduce infection rates,9 and is practised by >95% of operators for trans-venous systems.10 Cephalosporins, penicillins and teicoplanin are commonly used, providing cover against Staphylococcus aureus, with the latter also being effective against methicillin-resistant Staphylococcus aureus (MRSA).11
Anaphylaxis following teicoplanin administration is extremely rare. We present two cases of fatal anaphylaxis following teicoplanin administration during CIED procedures.
Case one
An 80-year-old woman attended for elective pacemaker generator change plus new atrial lead, due to elective replacement indicator and a rising atrial threshold. She had a dual-chamber pacemaker implanted 14 years previously for second-degree atrioventricular (AV) nodal block and a successful generator replacement five years later. She had previously received flucloxacillin, vancomycin and gentamycin without complication. She had a past medical history of asthma and a documented sensitivity to penicillin (nausea). Prior to commencing her procedure, a venogram was undertaken using non-ionic contrast. Approximately five minutes later her skin was prepared with chlorhexidine and intravenous paracetamol, teicoplanin and subcutaneous bupivacaine were administered. Almost immediately, she complained of feeling nauseated and generally unwell. Systolic blood pressure dropped from 150 mmHg to 94 mmHg and she was noted to look flushed. Chlorpheniramine, hydrocortisone, intravenous saline and oxygen were administered as per UK advanced life support guidelines.12 She became increasingly hypotensive, unresponsive and then pulseless. Full cardiopulmonary resuscitation (CPR) was instituted and she was intubated. During 90 minutes of CPR a total of 12 mg of intravenous adrenaline were administered and an infusion of dobutamine commenced. There was transient, brief return of spontaneous circulation (RoSC) followed by recurrent cardiac arrest with pulseless electrical activity (PEA). CPR was discontinued after 90 minutes in view of futility. Mast cell tryptase taken during CPR was elevated at 26.2 µg/L (normal range 2–14 µg/L) and cause of death was certified following post-mortem examination as anaphylaxis, most likely due to teicoplanin.
Case two
An 81-year-old woman presented to the emergency department with several episodes of pre-syncope. Past medical history included hypertension, osteoporosis and lumbar radiculopathy. A 12-lead electrocardiogram (ECG) demonstrated sinus bradycardia and trifascicular block. While in the department, she experienced further symptomatic episodes correlating with bradycardia. She had multiple known allergies and sensitivities: quinine (intolerance), angiotensin-converting enzyme (ACE) inhibitors (unspecified), candesartan (blurred vision), aspirin (unspecified), alendronic acid (vomiting), co-dydramol (intolerance) and nitrofurantoin (lip swelling). Her family also reported a non-specific intolerance to penicillin. In view of her symptoms and associated trifascicular block she was offered a permanent pacemaker. Her skin was prepared with chlorhexidine, and teicoplanin, fentanyl, midazolam and ondansetron were administered intravenously. She had previous documented exposure to fentanyl and benzodiazepines without allergy. Within two minutes of drug administration she became acutely agitated and complained of back pain, limb pain, nausea and dyspnoea. Non-invasive blood pressure and peripheral oxygen saturations were unrecordable, cardiac monitoring was normal throughout. There was no evidence of urticaria, angioedema or wheeze. Oxygen, adrenaline, hydrocortisone and chlorpheniramine were administered, but cardiac arrest occurred within one minute (PEA). Immediate CPR was commenced and the patient was intubated and ventilated. Further boluses of adrenaline, salbutamol, naloxone, flumazenil and intravenous fluids were administered. There was one brief episode of RoSC during which she was hypotensive, agitated and complained of dyspnoea. After one hour, CPR was abandoned in view of futility. Mast cell tryptase was significantly elevated at 71.5 µg/L. Cause of death was issued as anaphylaxis to teicoplanin following post-mortem examination.
Discussion
Anaphylaxis is a systemic hypersensitivity reaction characterised by histamine release via degranulation of mast cells and basophils. Symptoms and signs of anaphylaxis can affect any organ, although most commonly the skin (88%), respiratory (76%), cardiovascular (42%) and gastrointestinal (13%) systems.13 The incidence of hospitalisation for anaphylaxis is rising,14 and, over a lifetime, the prevalence is estimated at 0.05–2%.15 In adults, drugs cause the majority of reactions,16 most commonly non-steroidal anti-inflammatory drugs and beta-lactam antibiotics.13
Anaphylaxis following teicoplanin is extremely rare. The first reported case of anaphylactic shock was reported in 2006,17 with a further report in 2014.18 The first case series from two allergy clinics identified seven ‘definite’, seven ‘probable’ and two ‘uncertain’ cases of anaphylaxis due to teicoplanin.19
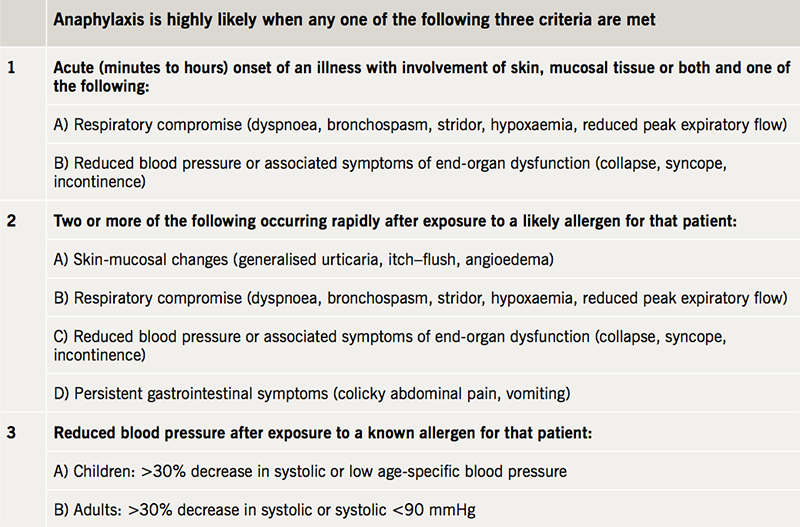
The case reported by Warrener et al.18 noted angioedema and cardiovascular collapse only, whereas the majority of cases reported by Savic et al.19 noted either angioedema or bronchospasm. Both of our cases were characterised by cardiovascular collapse minutes following teicoplanin administration. Importantly, there was an absence of urticaria, angioedema or bronchospasm in both cases, despite skin and respiratory changes being the most frequently reported. Our first case was noted to be flushed and our second complained of dyspnoea. Another possible explanation would be non-immunological mast cell activation, which has previously been reported with vancomycin, another glycopeptide antibiotic, and resulting in red man syndrome.20 This is often related to the speed of infusion (in both of our cases the dose was given rapidly as a bolus over 2–4 minutes), although it is extremely uncommon with teicoplanin,21 and there was also an absence of generalised erythema.20 Both of our cases meet the 2011 definition of anaphylaxis published by the World Allergy Organisation, which has a diagnostic sensitivity of 97% and specificity of 82% (table 1).22,23 Mast cell tryptase levels were also significantly elevated, which has previously been reported to be 93% specific for anaphylaxis.24 Unfortunately, in both of our cases we were unable to ascertain whether patients had received exposure to teicoplanin previously.
Conclusion
These cases serve to highlight the importance of anaphylaxis to teicoplanin, presenting as cardiovascular collapse, as a procedural complication. Despite early recognition and prompt treatment, this severe reaction was fatal in both cases at our centre. In view of the potential for non-immunological mast cell activation, caution should be undertaken when infusing teicoplanin rapidly. Operators should be aware of this risk, as it seems likely that these may become more frequent in an era of increasing implant rates, re-intervention rates and a rising incidence in anaphylaxis.14
Conflicts of interest
None declared.
Funding
None.
Consent
Patient consent for publication for both cases was obtained from the next of kin.
References
1. British Heart Rhythm Society. National audit of cardiac rhythm management devices. April 2015–March 2016. London: NICOR, 2017. Available from: http://www.bhrs.com/audit/ [accessed 13 November 2017]
2. Voigt A, Shalaby A, Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol 2010;33:414–19. https://doi.org/10.1111/j.1540-8159.2009.02569.x
3. Reynolds MR, Cohen DJ, Kugelmass AD et al. The frequency and incremental cost of major complications among medicare beneficiaries receiving implantable cardioverter-defibrillators. J Am Coll Cardiol 2006;47:2493–7. https://doi.org/10.1016/j.jacc.2006.02.049
4. Tarakji KG, Chan EJ, Cantillon DJ et al. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm 2010;7:1043–7. https://doi.org/10.1016/j.hrthm.2010.05.016
5. Padfield GJ, Steinberg C, Bennett MT et al. Preventing cardiac implantable electronic device infections. Heart Rhythm 2015;12:2344–56. https://doi.org/10.1016/j.hrthm.2015.06.043
6. Sohail MR, Uslan DZ, Khan AH et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol 2007;49:1851–9. https://doi.org/10.1016/j.jacc.2007.01.072
7. Prutkin JM, Reynolds MR, Bao H et al. Rates of and factors associated with infection in 200 909 Medicare implantable cardioverter-defibrillator implants: results from the National Cardiovascular Data Registry. Circulation 2014;130:1037–43. https://doi.org/10.1161/CIRCULATIONAHA.114.009081
8. Sandoe JA, Barlow G, Chambers JB et al. Guidelines for the diagnosis, prevention and management of implantable cardiac electronic device infection. Report of a joint Working Party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). J Antimicrob Chemother 2015;70:325–59. https://doi.org/10.1093/jac/dku383
9. Da Costa A, Kirkorian G, Cucherat M et al. Antibiotic prophylaxis for permanent pacemaker implantation: a meta-analysis. Circulation 1998;97:1796–801. https://doi.org/10.1161/01.CIR.97.18.1796
10. Basil A, Lubitz SA, Noseworthy PA et al. Periprocedural antibiotic prophylaxis for cardiac implantable electrical device procedures: results from a Heart Rhythm Society survey. JACC Clin Electrophysiol 2017;3:632–4. https://doi.org/10.1016/j.jacep.2017.01.013
11. Svetitsky S, Leibovici L, Paul M. Comparative efficacy and safety of vancomycin versus teicoplanin: systematic review and meta-analysis. Antimicrob Agents Chemother 2009;53:4069–79. https://doi.org/10.1128/AAC.00341-09
12. Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. London: Resuscitation Council (UK), January 2008. Available from: https://www.resus.org.uk/anaphylaxis/emergency-treatment-of-anaphylactic-reactions/ [accessed 26 February 2018].
13. Montanez MI, Mayorga C, Bogas G et al. Epidemiology, mechanisms, and diagnosis of drug-induced anaphylaxis. Front Immunol 2017;8:614. https://doi.org/10.3389/fimmu.2017.00614
14. Turner PJ, Gowland MH, Sharma V et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992–2012. J Allergy Clin Immunol 2015;135:956.e1–963.e1. https://doi.org/10.1016/j.jaci.2014.10.021
15. Lieberman P, Camargo CA Jr., Bohlke K et al. Epidemiology of anaphylaxis: findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann Allergy Asthma Immunol 2006;97:596–602. https://doi.org/10.1016/S1081-1206(10)61086-1
16. Gonzalez-Perez A, Aponte Z, Vidaurre CF, Rodriguez LA. Anaphylaxis epidemiology in patients with and patients without asthma: a United Kingdom database review. J Allergy Clin Immunol 2010;125:1098.e1–1104.e1. https://doi.org/10.1016/j.jaci.2010.02.009
17. Asero R. Teicoplanin-induced anaphylaxis. Allergy 2006;61:1370. https://doi.org/10.1111/j.1398-9995.2005.01021.x
18. Warrener T, Southall P, Kapur S. Anaphylactic shock secondary to teicoplanin. Anaesthesia 2014;68(s3):15. Available from: https://www.anaesthesiacases.org/case-reports/2014-0003
19. Savic LC, Garcez T, Hopkins PM, Harper NJ, Savic S. Teicoplanin allergy – an emerging problem in the anaesthetic allergy clinic. Br J Anaesth 2015;115:595–600. https://doi.org/10.1093/bja/aev307
20. Sivagnanam S, Deleu D. Red man syndrome. Crit Care 2003;7:119–20. https://doi.org/10.1186/cc1871
21. Wilson AP. Comparative safety of teicoplanin and vancomycin. Int J Antimicrob Agents 1998;10:143–52. https://doi.org/10.1016/S0924-8579(98)00025-9
22. Simons FE, Ebisawa M, Sanchez-Borges M et al. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J 2015;8:32. https://doi.org/10.1186/s40413-015-0080-1
23. Campbell RL, Li JT, Nicklas RA, Sadosty AT. Emergency department diagnosis and treatment of anaphylaxis: a practice parameter. Ann Allergy Asthma Immunol 2014;113:599–608. https://doi.org/10.1016/j.anai.2014.10.007
24. Brown SG, Blackman KE, Heddle RJ. Can serum mast cell tryptase help diagnose anaphylaxis? Emergency Medicine Australas 2004;16:120–4. https://doi.org/10.1111/j.1742-6723.2004.00562.x
