Cardiac catheterisation is a common invasive procedure. Transradial vascular access is the default approach due to a reduced risk of vascular and bleeding complications. Although transradial vascular access complications are infrequent it is important to identify, mitigate and manage them appropriately when they arise. Several techniques have been identified to try to reduce their occurrence pre- and post-procedurally, as well as manage any complication sequalae. This review article summarises the incidence, type, prevention and management of complications encountered in transradial vascular access.
Introduction
Introduced by Dr Lucien Campeau in 1989, transradial vascular access (TRA) is now the standard approach for diagnostic coronary angiography due to a reduced incidence of complications compared with femoral access, increased patient satisfaction, a quicker recovery time and a reduction in mortality in those with ST-elevation myocardial infarction (STEMI).1 Radial access is associated with a 77% reduction in major vascular complications compared with transfemoral access, and is, therefore, recommended as the default access for patients presenting with acute coronary syndromes in current European Society of Cardiology (ESC) guidelines.2
There are, however, important potential complications associated with TRA. These include both intra-procedural complications, such as radial artery spasm or perforation, and post-procedural complications, such as compartment syndrome or radial arterio-venous fistula. This article aims to discuss the incidence, risk factors and management of these complications, which are especially important to know when discussing consent for angiographic procedures.
Radial access complications
Vascular complications following transradial artery access are uncommon. Major complications occur in about 0.2% of cases and significant bleeding complications in about 1% of cases.
Complications of radial access can be divided into intra-procedural and post-procedural:1 these are detailed in table 1.
Table 1. Complications of transradial access and their incidence
| Intra-procedural | Incidence |
|---|---|
| Radial artery spasm (RAS) and equipment entrapment | ~5% (severe 2.7%, entrapment <1%) |
| Radial artery perforation/dissection | <1% |
| Catheter kinks | <1% |
| Vasovagal events | 6% |
| Post-procedural | |
| Access-site haematoma | 2.6% |
| Compartment syndrome | 0.004% |
| Radial artery occlusion without adverse sequelae | 1–33% |
| Pseudoaneurysm | 0.06% |
| Arteriovenous fistulae | <0.01% |
Intra-procedural complications
Radial artery spasm
Radial artery spasm (RAS) is the abrupt, spasmodic narrowing of the radial artery. Quoted incidence ranges widely from 4 to 20%,1 with larger trials suggesting about 5%.3 RAS occurs due to traumatic irritation of the artery, which is highly vasoactive, containing a high number of alpha-adrenoreceptors in the adventitia. This leads to localised spasm at the site of trauma.4
Clinically, RAS presents as pain or discomfort for the patient, with increased resistance while manipulating the sheath, wire or catheter. The majority of events are mild, but severe spasm (2.7%)5 can occasionally lead to more significant complications, including catheter entrapment (<1%) or, very rarely, radial eversion endarterectomy.1 Several techniques may be employed to minimise the risk of RAS.
- Selection of suitable patients is important. Risk factors for RAS include female gender, small arterial diameter (<2 mm),6 arterial anomaly seen on ultrasound, previous access failure and vaso-occlusive disease (e.g. vasculitis or Raynaud’s phenomenon). If there are multiple risk factors present, femoral access may be preferred.
Routine ultrasound can be used to assess the artery for size and aberrant anatomy. The most common anomaly seen is a high-bifurcation radial origin (7%) associated with a small diameter vessel (<3 mm). Radial artery loops (2.3%) and severe tortuosity (2%) are occasionally found: these are rarer but associated with a significantly higher failure rate (37% and 23%, respectively) than high origin bifurcation (5%). If identified, the use of hydrophilic wires (e.g. Terumo Glidewire) can be used to navigate cautiously during the procedure. - Consideration of pre-procedural sedation (e.g. midazolam) and analgesia (e.g. fentanyl) in higher risk patients. Studies have shown that patients given sedation and analgesia have a significantly lower (2.6% vs. 8.3%) occurrence of RAS.3
- Intra-procedural techniques. Adequate local anaesthetic use can help to reduce the risk of RAS. Administration of glyceryl trinitrate (GTN) sublingually or topically/subcutaneously at the site of intended access dilates the artery allowing for easier palpation and visualisation on ultrasound. Subcutaneous GTN has been shown to reduce the risk of RAS (1% vs. 8% without),7 but there is less evidence for sublingual and topical use. There are contraindications to GTN use, such as severe aortic stenosis, hypertrophic cardiomyopathy and hypotension.
- While gaining access, ultrasound use during arterial puncture is especially helpful if there are small vessels, or to minimise repeated attempts if there is concern about challenging access. There is no evidence of benefit from routine ultrasound use over palpation alone.8 Puncture of the anterior arterial wall alone (Seldinger technique) minimises trauma.
- Sheath choice also affects RAS: long radial sheaths with the smallest possible diameter and hydrophilic coating give the best outcome. Evidence suggests that compared with non-coated sheaths, hydrophilic-coated sheaths reduce RAS incidence by about 50% (19% vs. 40%, odds ratio [OR] 2.87).9
- A vasodilatory intra-arterial cocktail can also help to minimise RAS. Nitrates (e.g. isosorbide dinitrate, GTN) and calcium-channel blockers (CCBs, e.g. verapamil, diltiazem, nicardipine) are used alone or in combination. The American Heart Association (AHA) endorses the use of a CCB (verapamil/diltiazem 2.5–5 mg) with GTN (100–200 µg) at sheath insertion ± at removal.10
- If RAS occurs prior to sheath insertion, it can be reduced by heating of the radial site (either palm-mediated or a warm compress with towels), blood pressure (BP) cuff inflation for three minutes at 30 mmHg above systolic pressure to cause reactive vasodilation, or subcutaneous GTN.
Management of RAS
Management of RAS depends on the degree of spasm. Initially, the anatomy should be assessed with gentle contrast injection. This can allow the site of spasm to be identified, and exclude vascular abnormalities, arterial dissection, or perforation. Mild spasm can be treated by sedation and analgesia, and further intra-arterial spasmolytic therapy.
More severe RAS can be associated with equipment entrapment. Techniques for mild spasm should first be attempted. If these are unsuccessful then forearm heating, BP cuff vasodilation and subcutaneous nitrate injection at the site of spasm should be tried. In severe cases with catheter entrapment, ViperSlide lubricant can be used to remove entrapped equipment.11
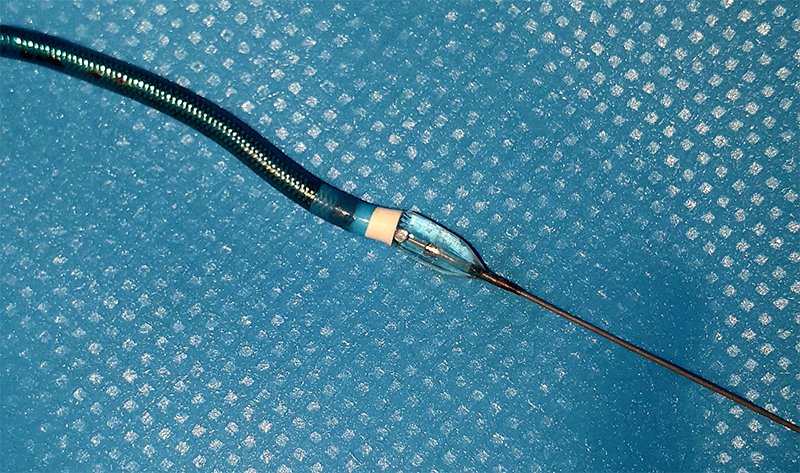
If initial interventions are unsuccessful, balloon-assisted tracking (BAT) can be used (figure 1). This involves expansion of a balloon half out of the catheter, which can help traverse the area of spasm and reduce the need for crossover. In cases where alternative options have failed, deep conscious sedation (propofol) or general anaesthesia can be used: this causes widespread sympathetic inhibition and vasodilation, facilitating equipment removal. Alternatively, axillary, brachial plexus or radial nerve block can provide a temporary functional sympathectomy and allow sufficient vasodilation to remove entrapped equipment. However, this is not well studied in anticoagulated patients and should be done as a last resort. If all non-invasive techniques are exhausted, surgical endarterectomy should be considered. After managing severe RAS, crossover to femoral access should be considered, depending on the indication and urgency of the case.
If the very rare, but serious, complication of radial artery endarterectomy or avulsion occurs on sheath removal, a vascular surgical opinion should be sought as an emergency.
Radial artery perforation
Radial artery perforation is an uncommon complication of radial artery access seen in <1% of procedures.12 It is often associated with wire advancement into a small arterial side branch causing trauma, perforation or avulsion. Artery dissection without perforation can also occur, and has the same risk factors and management.
Techniques to reduce the risk of radial artery perforation include careful advancement of the J wire: if any resistance or patient discomfort occurs, angiography should be used to assess for abnormal anatomy or complications. Occasionally, the advancement of the catheter, rather than the wire, causes arterial injury and perforation due to the razor effect – the stiff edge of the catheter slicing into the arterial wall. To reduce this risk, balloon-assisted tracking can be used to overcome coronary spasm, tortuosity and loops, as well as reducing the risk of dissection/perforation.
Management of radial artery perforation
Management of radial artery perforation is dependent on whether the wire is distal or proximal to where the perforation has occurred. If the wire is proximal, it is usually possible to seal the perforation with either a longer sheath or guide catheter. Often the procedure can be completed and the perforation site reassessed with a final angiogram. If the perforation persists then management should include protamine administration to reverse peri-procedural heparin, BP cuff inflation proximal to the perforation, and discussion with the vascular surgical team. Interventional options can include intra-arterial prolonged balloon inflation (up to 20 minutes), use of a covered stenting or vascular surgery.
If the wire is distal to the site of perforation, cautious attempts can be made to cross the site and allow for balloon inflation or use of a covered stenting. Protamine and BP cuff compression should then be undertaken as before, and further management based on discussion with surgical colleagues.
Catheter kinks
Catheter kinks are generated by excessive manipulation and torque of the catheter during the procedure. This can be due to tortuous anatomy or entrapment due to spasm. It can be readily identified by sudden loss of the aortic pressure tracing. Screening of the entire catheter length should then be undertaken to look for kinks.
If a kink is identified, the catheter must be untwisted. Gentle advancement of the 035 guide wire can be attempted. If this fails, then distal fixation of the catheter, which can be achieved by BP cuff inflation if the kink is in the forearm, can allow for untwisting of the knot. Alternative techniques are to use a long sheath to engulf or straighten the kink, or use of a snare introduced via femoral access to grasp the kinked catheter allowing rotation of both ends to untwist the kink.13
Vasovagal events
Vagal stimulation is reported to occur in 6% of cardiac catheterisation cases.14 Indicated by bradycardia and hypotension, with the potential for atrioventricular block. Incidence can be reduced by adequate pre-procedural hydration. Mostly triggered by access site acquisition or sheath removal, in coronary angiography vasovagal reactions can specifically be triggered by catheter manipulation activating aortic arch/carotid baroreceptors or coronary intubation and contrast injection suppressing sinus node function (predominantly right coronary artery [RCA]). Management of intra-procedural events involves withdrawal of the noxious stimulation driving the vagal reaction (i.e. catheter manipulation/contrast injection), intravenous fluid bolus and chronotropic agents, such as atropine, to treat any significant bradycardia. Rarely, more invasive management, such as temporary pacing, is required for persistent bradyarrhythmia. It is important to exclude more serious causes for a sudden drop in blood pressure (i.e. haemorrhage, coronary artery dissection/occlusion or pericardial effusion) dependent on the clinical context.
Post-procedural complications
Access site haematoma
Clinically significant haematomas occur in approximately 1.2–2.6% of cases, but can be seen on ultrasound in 23%.15 Categorisation follows EASY trial grading:16 Grade I (<5 cm, local haematoma), Grade II (<10 cm, local with moderate muscular involvement), Grade III (<10 cm below elbow with moderate muscular involvement), Grade IV (above the elbow with moderate muscular involvement) and Grade V (anywhere with threat of compartment syndrome/ischaemia).
Compartment syndrome occurs very rarely (0.004%).17 Bleeding into soft tissues can increase intra-compartmental pressures, reducing arterial perfusion and venous return, which leads to ischaemia, tissue necrosis and further swelling. If untreated, muscle contractures, paralysis, deformity, rhabdomyolysis and, potentially, mortality can occur. Treatment often requires surgical fasciotomy.
Early detection of haematomas prevents progression and further complications. Small haematomas usually only require elevation of the limb and alteration of the radial compression device. If a larger haematoma is identified, then prompt BP cuff inflation to provide haemostasis with gradual release of pressure can be employed, or, alternatively, the use of a compression dressing covering the forearm.
Anticoagulation should be held and reviewed after 24 hours. Good control of hypertension and pain is important, and plethysmography should be used to monitor for development of compartment syndrome. If concerned, direct intra-compartmental pressure measurement should be undertaken with involvement of the surgical team.
Radial artery occlusion
Radial artery occlusion (RAO) has been reported with a wide range of incidence (1–33%),1 but with optimal preventative measures rates of <1% can be achieved.18 Most patients are asymptomatic, and symptomatic RAO requiring medical management is very uncommon (0.2%).19 It is useful to preserve radial artery patency for future angiography, graft conduit or fistulae formation, but occlusion has been shown to have no impact on functional outcomes or digital blood supply.20
RAO can be prevented by avoiding spasm, use of small sheaths, adequate intra-arterial anticoagulation, patient haemostasis of reduced (two-hour) duration and ipsilateral ulnar compression.17 Treatment with anticoagulation or percutaneous intervention can be offered if symptomatic, however, spontaneous recanalisation often occurs over time.
Pseudoaneurysm
Pseudoaneurysm is a rare complication (0.03–0.09%) that usually presents with a pulsatile swelling at the access site, sometimes with pain. Diagnosis is made using ultrasound. The need for closure is low (0.2%).21 Management can include compression, thrombin injection, covered stent or surgical repair.
Arteriovenous fistulae
Iatrogenic arteriovenous fistulae (AVF) are formed when simultaneous puncture of an adjacent artery and vein leads to a persistent communication. Femoral AVF are seen more commonly, especially following therapeutic catheterisation. Although much rarer, AVF following cardiac catheterisation using a radial approach are also reported (0–0.08%). Diagnosis is made by duplex ultrasound (figure 2).
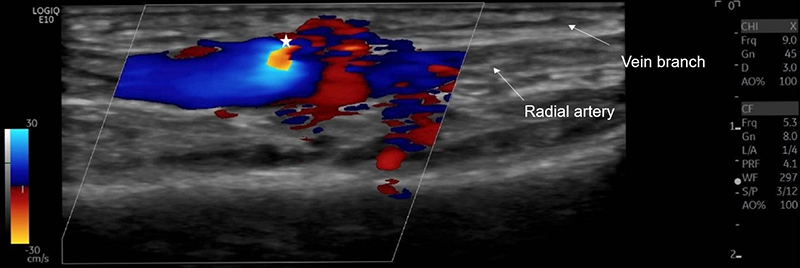
The risk of AVF can be reduced by experienced operators, using sheath sizes less than the arterial diameter, minimising repeated access and using ultrasound guidance. Typically, 5 or 6 FG sheaths are used for radial artery access. Using sheaths where the ratio of the inner radial artery diameter to the outer sheath diameter is >1.0 increases the risk of severe flow reduction in the artery from 4% to 13%, with an ideal ratio being 0.9.22
Distal transradial access
An emerging technique for vascular access, distal transradial access (dTRA) involves puncture of the radial artery on the dorsum of the hand at the anatomical snuff box. Compared with proximal radial access, dTRA has several proposed benefits including an ergonomic benefit to the patient and operator, association with lower incidence of RAO (0.91% vs. 0.31%) and shorter haemostasis time (c. 180 vs. 153 minutes). Limitations include increased incidence of RAS (2.7% vs. 5.4%), slightly longer access time and higher crossover rates (3.5% vs. 7.4%).23 It offers a viable option in cases where haemostasis or RAO is of concern, at the potential trade-off with higher crossover and RAS rates.
Contrast-induced nephropathy
Contrast-induced nephropathy (CIN) is an increase in serum creatinine post-contrast administration in the absence of an alternative explanation. It is seen in about 6% of patients undergoing angiography, and defined as a 25% relative increase or 0.5 mg/dL absolute increase of serum creatinine.24 Due to vasoconstrictive renal medullary hypoxia and direct contrast toxicity to tubular cells, it can rarely lead to the need for renal replacement therapy (0.5%).24 Risk factors include chronic kidney disease (especially estimated glomerular filtration rate [GFR] <40 ml/min), diabetes, dehydration, haemodynamic instability (requiring inotropic or mechanical support), prior CIN and large-volume contrast exposure (>4 ml/kg).24 Methods to attempt to prevent CIN in patients at risk include:25
- Discontinuation of nephrotoxic medications for 48 hours pre-procedure.
- Intravenous pre-hydration (0.9% saline 1–1.5 ml/kg for 12 hours or 3 ml/kg for one hour in elective cases).
- Intravenous post-hydration (0.9% saline for 1–1.5 ml/kg for 12–24 hours).
- Use of contrast agents that are isosmotic (i.e. Visipaque) with lowest possible volume use, including non-contrast assessment of coronary anatomy (i.e. intravascular ultrasound).
Repeat serum creatinine testing should be undertaken at 48–72 hours post-contrast exposure. If CIN is diagnosed, then it should be managed using recommended acute kidney injury (AKI) guidelines, such as those provided by the National Institute for Health and Care Excellence (NICE).26 Although contrast use is similar between femoral and radial access routes, incidence of AKI is about 34% lower with radial access, in part due to reduced incidence of bleeding events and athero-embolisation from femoral catheter transversal of thoraco-abdominal aorta.27
Conclusion
Transradial access is now the standard method for obtaining arterial access for coronary angiography. There are several preventive methods that can be employed to reduce the peri-procedural risk of complications. Although complications are rare, with early identification and prompt management, their incidence and severity can be decreased.
Key messages
- Transradial vascular access for cardiac catheterisation is the favoured approach due to shorter admissions and fewer complications
- It is important to detect transradial access complications early to allow for prompt, appropriate management. Delayed diagnosis can compound the severity of complications and lead to a requirement for surgical management
- Intra-procedural complications include radial artery spasm (RAS), perforation and catheter kinks
- Post-procedural complications include haematoma, radial artery occlusion, pseudoaneurysm and, rarely, arteriovenous fistulae
Conflicts of interest
None declared.
Funding
None.
Acknowledgement
We acknowledge radiologist Dr Ignotus for his contributions in US duplex image acquisition and interpretation.
References
1. Sandoval Y, Bell MR, Gulati R. Transradial artery access complications. Circ Cardiovasc Interv 2019;12:e007386. https://doi.org/10.1161/CIRCINTERVENTIONS.119.007386
2. Ibanez B, James S, Agewall S et al.; ESC Scientific Document Group. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–77. https://doi.org/10.1093/eurheartj/ehx393
3. Deftereos S, Giannopoulos G, Raisakis K et al. Moderate procedural sedation and opioid analgesia during transradial coronary interventions to prevent spasm: a prospective randomized study. JACC Cardiovasc Interv 2013;6:267–73. https://doi.org/10.1016/j.jcin.2012.11.005
4. Caputo RP, Tremmel JA, Rao S et al. Transradial arterial access for coronary and peripheral procedures: executive summary by the Transradial Committee of the SCAI. Catheter Cardiovasc Interv 2011;78:823–39. https://doi.org/10.1002/ccd.23052
5. Goldsmit A, Kiemeneij F, Gilchrist IC et al. Radial artery spasm associated with transradial cardiovascular procedures: results from the RAS registry. Catheter Cardiovasc Interv 2014;83:E32–E36. https://doi.org/10.1002/ccd.25082
6. Dieter RS, Akef A, Wolff M. Eversion endarterectomy complicating radial artery access for left heart catheterization. Catheter Cardiovasc Interv 2003;58:478–80. https://doi.org/10.1002/ccd.10441
7. Ezhumalai B, Satheesh S, Jayaraman B. Effects of subcutaneously infiltrated nitroglycerin on diameter, palpability, ease-of-puncture and pre-cannulation spasm of radial artery during transradial coronary angiography. Indian Heart J 2014;66:593–7. https://doi.org/10.1016/j.ihj.2014.05.023
8. Seto AH, Roberts JS, Abu-Fadel MS et al. Real-time ultrasound guidance facilitates transradial access: RAUST (Radial Artery access with Ultrasound Trial). JACC Cardiovasc Interv 2015;8:283–91. https://doi.org/10.1016/j.jcin.2014.05.036
9. Rathore S, Stables RH, Pauriah M et al. Impact of length and hydrophilic coating of the introducer sheath on radial artery spasm during transradial coronary intervention: a randomized study. JACC Cardiovasc Interv 2010;3:475–83. https://doi.org/10.1016/j.jcin.2010.03.009
10. Mason PJ, Shah B, Tamis-Holland JE et al. An update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome: a scientific statement from the American Heart Association. Circ Cardiovasc Interv 2018;11:e000035. https://doi.org/10.1161/HCV.0000000000000035
11. Fidone E, Price J, Gupta R. Use of ViperSlide lubricant to extract entrapped sheath after severe radial artery spasm during coronary angiography. Tex Heart Inst J 2018;45:186–7. https://doi.org/10.14503/THIJ-17-6394
12. Sanmartín M, Cuevas D, Goicolea J et al. Vascular complications associated with radial artery access for cardiac catheterization. Rev Esp Cardiol 2004;57:581–4. https://doi.org/10.1016/S0300-8932(04)77150-X
13. Ben-Dor I, Rogers T, Satler LF, Waksman R. Reduction of catheter kinks and knots via radial approach. Catheter Cardiovasc Interv 2018;92:1141–6. https://doi.org/10.1002/ccd.27623
14. Gedela M, Kumar V, Shaikh KA, Stys A, Tomasz S. Bradycardia during transradial cardiac catheterization due to catheter manipulation: resolved by catheter removal. Case Rep Vasc Med 2017;2017:8538149. https://doi.org/10.1155/2017/8538149
15. Riangwiwat T, Blakenshio JC. Vascular complications of transradial access for cardiac catheterization. US Cardiol 2021;15:e04. https://doi.org/10.15420/usc.2020.23
16. Bertrand OF, Larose E, Rodés-Cabau J et al. Incidence, predictors, and clinical impact of bleeding after transradial coronary stenting and maximal antiplatelet therapy. Am Heart J 2009;157:164–9. https://doi.org/10.1016/j.ahj.2008.09.010
17. Tizón-Marcos H, Barbeau GR. Incidence of compartment syndrome of the arm in a large series of transradial approach for coronary procedures. J Interv Cardiol 2008;21:380–4. https://doi.org/10.1111/j.1540-8183.2008.00361.x
18. Pancholy SB, Bernat I, Bertrand OF, Patel TM. Prevention of radial artery occlusion after transradial catheterization: the PROPHET-II randomized trial. JACC Cardiovasc Interv 2016;9:1992–9. https://doi.org/10.1016/j.jcin.2016.07.020
19. Jolly SS, Yusuf S, Cairns J et al.; RIVAL Trial Group. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet 2011;377:1409–20. https://doi.org/10.1016/S0140-6736(11)60404-2
20. Rymer JA, Rao SV. Preventing acute radial artery occlusion: a battle on multiple fronts. JACC Cardiovasc Interv 2018;11:2251–3. https://doi.org/10.1016/j.jcin.2018.09.018
21. Kim D, Orron DE, Skillman JJ et al. Role of superficial femoral artery puncture in the development of pseudoaneurysm and arteriovenous fistula complicating percutaneous transfemoral cardiac catheterization. Cathet Cardiovasc Diagn 1992;25:91–7. https://doi.org/10.1002/ccd.1810250203
22. Saito S, Ikei H, Hosokawa G et al. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Catheter Cardiovasc Interv 1999;46:173–8. https://onlinelibrary.wiley.com/doi/10.1002/%28SICI%291522-726X%28199902%2946%3A2%3C173%3A%3AAID-CCD12%3E3.0.CO%3B2-4
23. Aminian A, Sgueglia G, Wiemer M et al. Distal versus conventional radial access for coronary angiography and intervention. JACC Interv 2022;15:1191–201. https://doi.org/10.1016/j.jcin.2022.04.032
24. Rear R, Bell RM, Hausenloy DJ. Contrast-induced nephropathy following angiography and cardiac interventions. Heart 2016;102:638–48. https://doi.org/10.1136/heartjnl-2014-306962
25. Windecker S, Kolh P, Alfonso F et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014;35:2541–619. https://doi.org/10.1093/eurheartj/ehu278
26. National Institute for Health and Care Excellence. Acute kidney injury: prevention, detection and management. NG148. London: NICE, 2019. Available from: https://www.nice.org.uk/guidance/ng148
27. Wang C, Chen W, Yu M, Yang P. Comparison of acute kidney injury with radial vs. femoral access for patients undergoing coronary catheterization: an updated meta-analysis of 46,816 patients. Exp Ther Med 2020;20:42. https://doi.org/10.3892/etm.2020.9170
