At least 5% of GP and accident and emergency (A&E) attendances are undifferentiated chest pain. Rapid access chest pain clinics (RACPC) offer urgent guideline-directed management of suspected cardiac chest pain. The National Institute for Health and Care Excellence (NICE) recommends computed tomography coronary angiography (CTCA) as a first-line investigation. We evaluated the effectiveness and efficiency of a local RACPC.
Retrospective analysis of unselected referrals to a RACPC in the Northeast of England was conducted for 2021. Baseline demographics and major adverse cardiovascular events (MACE) were compared between typical, atypical and non-angina. Anatomical and functional imaging results were recorded. Backward stepwise binary logistic regression modelled obstructive coronary artery disease (CAD) incidence.
There were 373/401 (93.0%) patients with chest pain; 139 (37.3%) typical angina, 122 (32.8%) atypical angina and 112 (30.0%) non-angina. Typical angina patients were older (p<0.001) with more cardiovascular risk factors (p<0.001) and increased risk of obstructive CAD (adjusted odds ratio [OR] 6.27, 95% confidence interval [CI] 2.93 to 13.38) and MACE (9.4%, p=0.029). In total, 164 (44.0%) had invasive coronary angiography (ICA) within 7.4 ± 4.8 weeks; 19.5% had normal coronary arteries, 26.2% had obstructive CAD and 22.6% proceeded to invasive haemodynamic assessment ± PCI without major procedural complications. There were 39 (10.5%) who had CTCA within 34.6 ± 18.1 weeks; 25.6% needed ICA to clarify diagnosis.
In conclusion, typical angina patients were at heightened risk of cardiovascular events. In the absence of adequate CTCA capacity, greater reliance on ICA still facilitated accurate diagnosis with options for immediate revascularisation, timely and safely, in the right patients. Better risk stratification and expansion of non-invasive imaging can improve local RACPC service delivery in the wider Northeast cardiology network.
Introduction
Undifferentiated chest pain places a significant burden on the UK National Health Service (NHS). Up to 50% of the general population experiences chest pain in their lifetime contributing to at least 1% of GP consultations and 5% of accident and emergency (A&E) attendances.1 Chest pain patients have a twofold higher mortality versus age-matched asymptomatic controls.1 One reason is undiagnosed obstructive coronary artery disease (CAD), which has effective treatments to prolong life and improve symptoms.1 The challenge is identifying the patients at greatest risk, providing a timely diagnosis and starting effective treatment.
The national service framework for coronary heart disease set out a 10-year strategy to improve the care and outcomes of coronary heart disease patients in 2000.2 Part of the mandate was the establishment of rapid access chest pain clinics (RACPC) to provide expert cardiology consultation for patients with chest pain of suspected cardiac origin within two weeks of referral.2 An important advantage is the access to specialist cardiac tests to investigate CAD. In 2016, the revised guidelines from the National Institute of Health and Care Excellence (NICE) recommended first-line computed tomography (CT) coronary angiography (CTCA) in patients without known CAD, presenting with typical angina, atypical angina or non-angina chest pain, but where clinical suspicion of cardiac ischaemia remains.1,3
Reasons in favour of CTCA included relatively low cost and less radiation, a strong negative-predictive value and the prospect of improved medical management of non-obstructive atheromatous plaque.4 However, the provision of a national CTCA service would require access to more high-quality CT scanners with greater slice frequency and prospective gating, higher numbers of appropriately trained clinical staff and a geographically more equitable service delivery.5
The aim of our service evaluation was to assess the use of anatomical and functional imaging modalities to investigate angina at a RACPC against NICE recommendations. We wished to better understand the epidemiology and risk level of the RACPC population to make informed recommendations on the local triage system and provision of investigations for obstructive CAD.
Method
Study population and design
Patients referred to the RACPC at Sunderland Royal Hospital (SRH), a district general hospital in the Northeast of England in 2021 were retrospectively identified. Data were collected from three distinct periods: January to February, April to May and August to October 2021.
Eligible patients presented with typical, atypical or non-angina chest pain. Typical angina was defined by a constricting/heavy discomfort in the front of the chest, neck, shoulders, jaws or arms; worsened by physical activity; and relieved by rest or glyceryl trinitrate spray within five minutes.3 Any two of the three features defined atypical angina and one or no features characterised non-angina chest pain.3 Exclusion criteria were symptoms other than chest pain.
Patients were stratified by presenting complaint into typical angina, atypical angina or non-angina. Another important comparison was drawn between patients with ‘normal coronaries’, non-obstructive CAD and obstructive CAD. Obstructive CAD was defined by visually assessed ≥70% stenosis of the three epicardial coronary arteries (the left anterior descending [LAD], the left circumflex [LCx] and the right coronary artery [RCA]), ≥50% stenosis of the left main coronary artery (LMCA) and/or fractional flow reserve (FFR) or ctFFR positive (≤0.80) moderate lesions. Non-obstructive CAD ranged from minor and eccentric plaque disease to moderate stenoses of 50–69%.6
Data were collected from the local hospital’s electronic patient records, MediTech 6.0. Ethical approval was waived as anonymised retrospective data were used.
Variables of interest were patient age, sex, cardiovascular risk factors, clinical observations and signs, investigations and prescription of cardiac medication. Relevant investigations were electrocardiogram (ECG), echocardiogram and levels of haemoglobin (g/L), creatinine (μmol/L), estimated glomerular filtration rate (ml/min/1.73 m2), total and non-high-density lipoprotein (non-HDL) cholesterol (mmol/L). Important medications were lipid-lowering therapy, antithrombotic and anti-anginal agents. The imaging modality to investigate CAD was also recorded.
End points
The primary composite end point of major adverse cardiovascular events (MACE) included all-cause death, myocardial infarction (MI), or ischaemia-driven repeat revascularisation at maximum follow-up. In line with the fourth universal definition, MI was diagnosed by a rise in cardiac troponin (cTn) above the 99th percentile with evidence of ischaemia from symptoms, ECG changes, imaging and/or angiography.7 Ischaemia-driven revascularisation included any percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG) for refractory or unstable angina.
Additional secondary end points were the components of the primary end point, re-referral to cardiology and hospital re-attendance.
Statistical analysis
Numerical variables were reported as mean ± standard deviation (SD) for parametric data, median (interquartile range [IQR]) for non-parametric data and compared using either analysis of variance (ANOVA) or the Kruskal-Wallis test for multiple group comparisons. Categorical variables were reported as number (percentage) and compared using the Pearson’s chi-square test.
We modelled the incidence of obstructive CAD using binary logistic regression. Significant variables on univariate analysis were retained for multi-variate analysis and regressed against obstructive CAD using backward stepwise methodology.
Statistical analysis was performed on IBM SPSS version 2020. Statistical significance was assessed at the conventional two-sided 5% level (p<0.05).
Results
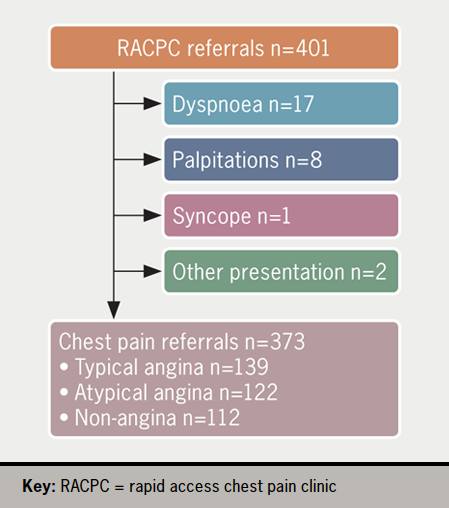
From 401 sampled RACPC referrals in 2021, 373 were due to chest pain, including 139 (37.3%) typical angina, 122 (32.8%) atypical angina and 112 (30.0%) non-angina (figure 1).
Patient characteristics
Typical angina patients were older (65.1 ± 11.4 vs. 61.1 ± 13.1 and 57.3 ± 15.2 years) and more likely to have major cardiovascular risk factors, including hypertension, diabetes, hypercholesterolaemia and a positive family history, compared with patients presenting with atypical angina and non-angina (table 1). Typical angina presentations were associated with higher rates of known CAD (28.1% vs. 16.4% and 10.7%) and abnormal echocardiograms (33.3% vs. 10.0% and 15.4%) than atypical angina and non-angina presentations. There was a trend to lower total cholesterol levels in patients with typical angina (4.6 ± 1.2 vs. 4.8 ± 1.2 and 3.5 ± 1.0 mmol/L).
Primary and secondary end points
The mean length of follow-up was 61.3 ± 16.7 weeks. The primary composite end point of MACE occurred in 13/139 (9.4%) patients with typical angina in comparison to 3/122 (2.5%) and 4/112 (3.6%) patients with atypical and non-angina (p=0.029). There was no difference in the secondary outcomes between patients presenting with typical, atypical and non-angina chest pain (p>0.05) (table 2).
Table 1. Baseline clinical characteristics
| Typical angina (n=139) | Atypical angina (n=122) | Non-angina (n=112) | p value | |
|---|---|---|---|---|
| Age, years | 65.1 ± 11.4 | 61.1 ± 13.1 | 57.3 ± 15.2 | <0.001 |
| Male | 82 (59.0) | 58 (47.5) | 57 (50.9) | 0.16 |
| Current smoker | 23 (16.5) | 19 (15.6) | 13 (11.6) | 0.52 |
| Hypertension | 85 (61.2) | 62 (50.8) | 42 (37.5) | <0.001 |
| Diabetes mellitus | 36 (25.9) | 20 (16.4) | 13 (11.6) | 0.011 |
| Hypercholesterolaemia | 102 (73.4) | 69 (56.6) | 53 (47.3) | <0.001 |
| Obesity | 50 (36.0) | 45 (36.9) | 29 (25.9) | 0.14 |
| Known CAD | 39 (28.1) | 20 (16.4) | 12 (10.7) | 0.002 |
| FH of CAD | 69 (49.6) | 64 (52.5) | 40 (35.7) | 0.023 |
| Known to cardiology | 17 (12.2) | 13 (10.7) | 13 (11.6) | 0.92 |
| Previous echocardiogram | 39 (28.1) | 30 (24.6) | 26 (23.2) | 0.66 |
| LVSD and/or RWMA | 13 (33.3) | 3 (10.0) | 4 (15.4) | 0.044 |
| Referral ECG available | 69 (49.6) | 58 (47.5) | 58 (51.8) | 0.81 |
| Clinic ECG done | 126 (90.6) | 103 (84.4) | 107 (95.5) | 0.017 |
| BBB pattern | 5 (4.0) | 4 (3.9) | 6 (5.6) | |
| ST changes | 5 (4.0) | 2 (1.9) | 1 (0.9) | |
| T-wave changes/q-waves | 19 (15.1) | 12 (11.7) | 9 (8.4) | |
| Sinus tachycardia/AF/ectopics | 14 (11.1) | 13 (12.6) | 11 (10.3) | |
| Heart rate, bpm* | 77 ± 14 | 75 ± 13 | 76 ± 12 | 0.50 |
| Systolic BP, mmHg* | 138 ± 20 | 135 ± 19 | 134 ± 17 | 0.46 |
| Murmur* | 6 (5.7) | 7 (8.3) | 8 (9.1) | 0.63 |
| Haemoglobin, g/L* | 139 ± 17 | 142 ± 15 | 141 ± 15 | 0.27 |
| Creatinine, μmol/L* | 86 ± 22 | 81 ± 23 | 84 ± 24 | 0.28 |
| eGFR, ml/min/1.73 m2* | 73 ± 15 | 77 ± 15 | 77 ± 14 | 0.11 |
| Total cholesterol, mmol/L* | 4.6 ± 1.2 | 4.8 ± 1.2 | 4.9 ± 1.0 | 0.05 |
| Non-HDL-C, mmol/L* | 3.2 ± 1.2 | 3.4 ± 1.2 | 3.5 ± 1.0 | 0.14 |
| Data are in mean ± standard deviation (SD) for numerical variables and n (%) for categorical variables unless otherwise stated. *>5% missing data. Key: AF = atrial fibrillation; BBB = bundle branch block; BP = blood pressure; CAD = coronary artery disease; ECG = electrocardiogram; eGFR = estimated glomerular filtration rate; FH = family history; LVSD = left ventricular systolic dysfunction; non-HDL-C = non-high-density lipoprotein cholesterol; RWMA = regional wall motion abnormality |
||||
Table 2. Patient outcomes
| Typical angina (n=139) | Atypical angina (n=122) | Non-angina (n=112) | p value | |
|---|---|---|---|---|
| Primary composite end point MACE | 13 (9.4) | 3 (2.5) | 4 (3.6) | 0.029 |
| Secondary end points | ||||
| All-cause death | 6 (4.3) | 2 (1.6) | 3 (2.7) | 0.43 |
| Myocardial infarction | 3 (2.2) | 0 (0.0) | 1 (0.9) | 0.23 |
| Ischaemia-driven revascularisation | 5 (3.6) | 1 (0.8) | 0 (0.0) | 0.055 |
| Re-referral to cardiology | 6 (4.3) | 6 (4.9) | 5 (4.5) | 0.97 |
| Hospital re-attendance | 11 (7.9) | 10 (8.2) | 8 (7.1) | 0.95 |
| Data are n (%) unless otherwise shown. Key: MACE = major adverse cardiovascular event |
||||
Investigations, diagnosis and treatment
The cumulative diagnoses stratified by presenting complaint and choice of investigation are presented in figure 2.
Overall, 164 (44.0%) underwent invasive coronary angiography (ICA), 39 (10.5%) CTCA, 32 (8.6%) CT coronary artery calcium score (CTCAC) and 31 (8.3%) myocardial perfusion scan (MPS). The mean time to investigation was 7.4 ± 4.8, 34.6 ± 18.1, 10.5 ± 8.8 and 11.4 ± 8.7 weeks, respectively. Another 29 (7.8%) were investigated for non-ischaemic heart disease and 74 (19.8%) were discharged without follow-up.
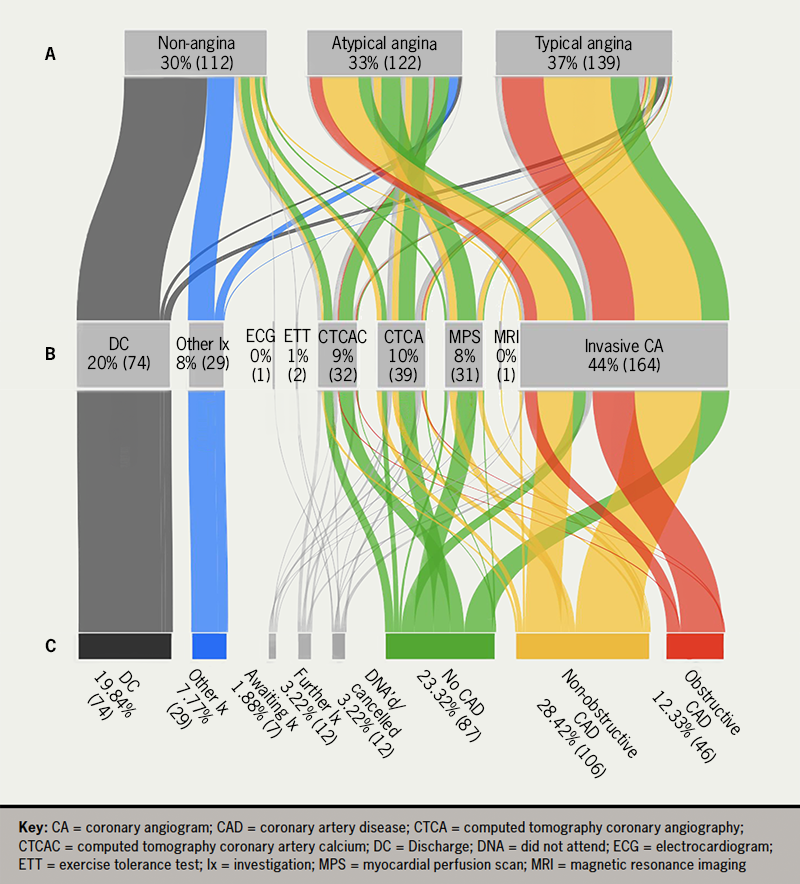
In total, 46 (12.3%) had obstructive CAD, 106 (28.4%) non-obstructive CAD and 87 (23.3%) normal coronary arteries. From 164 ICA, 32 (19.5%) had normal coronary arteries, 82 non-obstructive CAD (50.0%) and 43 (26.2%) obstructive CAD; 37 (22.6%) proceeded to FFR assessment and/or PCI. No major complications were reported. From 39 CTCA, 10 (25.6%) needed ICA to clarify diagnosis. Of the 31 MPS, two (6.4%) demonstrated reversible ischaemia and required ICA, which did not identify any stent targets.
At least 9 out of 10 patients with obstructive and non-obstructive CAD were prescribed primary and secondary prevention therapy before and after their diagnosis. Fewer than 50% of patients with normal coronary arteries were prescribed statin therapy after their diagnosis. We are unable to comment on whether this treatment was continued in the community.
Obstructive CAD risk
On univariate and multi-variate analysis, obstructive CAD was predicted by typical angina (odds ratio [OR] 6.27), age >61 years (OR 3.33), male sex (OR 2.09), known CAD (OR 2.64) and Hb >141 g/L (OR 2.83) (table 3).
Table 3. Univariate and multi-variate logistic regression to predict obstructive coronary artery disease (CAD)
| Unadjusted odds ratio | 95%CI | Adjusted odds ratio | 95%CI | |
|---|---|---|---|---|
| Typical angina | 6.82 | 3.33 to 13.97 | 6.27 | 2.93 to 13.38 |
| Age >61 years | 3.12 | 1.53 to 6.36 | 3.33 | 1.50 to 7.38 |
| Male | 2.85 | 1.43 to 5.70 | 2.09 | 0.94 to 4.61 |
| Current smoker | 1.18 | 0.47 to 2.92 | ||
| Hypertension | 2.21 | 1.15 to 4.25 | ||
| Diabetes mellitus | 1.67 | 0.82 to 3.43 | ||
| Hypercholesterolaemia | 1.81 | 0.92 to 3.56 | ||
| Obesity | 0.77 | 0.39 to 1.52 | ||
| FH of CAD | 1.07 | 0.58 to 1.98 | ||
| Known CAD | 3.72 | 1.93 to 7.18 | 2.64 | 1.27 to 5.53 |
| Abnormal ECG and/or echo | 1.78 | 0.91 to 3.47 | ||
| Hb >141 g/L | 1.96 | 1.03 to 3.71 | 2.83 | 1.31 to 6.09 |
| Creatinine >84 μmol/L | 2.61 | 1.39 to 4.92 | ||
| Total cholesterol >5 mmol/L | 0.67 | 0.34 to 1.32 | ||
| Data are n (%) unless otherwise shown. Key: CAD = coronary artery disease; CI = confidence interval; ECG = electrocardiogram; FH = family history; Hb = haemoglobin |
||||
Discussion
Non-specific chest pain is a common clinical presentation with an associated increased mortality rate that may be attributed to undiagnosed obstructive CAD.1–4 The RACPC aims to triage high-risk patients who require urgent intervention and intermediate-risk patients who need outpatient ischaemia testing from the bulk of low-risk ‘non-cardiac chest pain’ patients who can be safely discharged.1–4 In a service evaluation of a RACPC at a busy district general hospital in the Northeast of England, approximately 10% of patients had obstructive CAD. Typical angina patients were significantly more likely to have obstructive CAD with a higher one-year risk of MACE than other chest pain patients. Contrary to NICE recommendations, less than 10% had CTCA and instead, close to 50% of patients had ICA within two months of presentation, with a diagnostic rate of 20% normal coronary arteries and 25% obstructive CAD.
Similar to the populations of the CTCA in patients with suspected angina due to coronary heart disease (SCOT-HEART) trial and the Outcomes of Anatomical versus Functional Testing for Coronary Artery Disease (PROMISE) trial, our RACPC population was on average 60 years or older and 50% male with at least two modifiable cardiovascular risk factors.4,8 Our 37% typical angina burden was comparable with the 35% reported in SCOT-HEART.4
Our results are consistent with the published literature on the prognosis of patients presenting with and without typical angina.9–11 In an English multi-centre and a Scottish single-centre cohort study, angina patients had a 10–20% risk of a major cardiac event in comparison with less than 4% of patients with non-cardiac chest pain.10,11 We reported at least one in 10 patients with typical angina experiencing death of any cause, MI or ischaemia-driven revascularisation versus less than one in 20 patients with atypical or non-angina. The very low event rate among patients not presenting with typical angina should encourage stricter triaging of referrals. At least one fourth of chest pain patients could be safely discharged at triage.
In the SCOT-HEART trial, the addition of CTCA to the standard management of patients presenting with suspected angina clarified the diagnosis of CAD and angina, reduced further stress imaging, increased preventative therapy and was associated with reduced rates of cardiac death and MI at five years.4,12 Our high rate of ICA similarly improved our management of patients with suspected CAD.
There are likely to have been several reasons for our high ICA and low CTCA rate. First, a timely diagnosis is paramount. It can alleviate patient anxiety and pre-empt future cardiovascular events through appropriate treatment and intervention. In a cohort study of 172,180 first presentation chest pain patients, 4.6% of the 8,260 with a clinical diagnosis of angina experienced an MI in the first six months.9 Over 50% of our MACE events occurred within six months of RACPC attendance. On average, we provided ICA within two months of presentation versus CTCA within nine months. Second, ICA offers the opportunity to precisely quantify the haemodynamic significance of intermediate coronary lesions and to proceed directly to revascularisation in a ‘one-stop’ strategy in patients who remain symptomatic despite optimal medical anti-anginal therapy. One in five patients proceeded to FFR assessment and/or PCI after ICA.
The disadvantage of ICA in comparison with CTCA is the increased risk of serious complications including stroke, MI, contrast nephropathy and vascular access bleeding. Although we did not report any major complications, a significant number of ICA demonstrated normal coronary arteries. CTCA also facilitates better characterisation of plaque composition and risk stratification, which may be of greater relevance in a population at low risk of obstructive CAD.13 However, accurate interpretation is heavily dependent on patient factors (e.g. weight, heart rate control and breathing) and technical parameters (e.g. slice width and number, Z-axis coverage, reconstruction algorithms and ctFFR assessment).14 NICE recommends a minimum 64-slice CT scanner.3 Despite our use of a 128-slice CT scanner, a high proportion of patients who initially had CTCA required ICA to clarify diagnosis. The advantages of CTCA, including lower cost and less radiation were then obviated. A recently commissioned 320-slice CT scanner will be used in patients with sub-optimal heart rate control.
An alternative to anatomical imaging is non-invasive functional testing. The 2019 European Society of Cardiology (ESC) and the 2021 American Heart Association (AHA) guidelines on stable angina recommended non-invasive functional imaging to investigate stable CAD in intermediate-to-high risk patients, including those ≥65 years of age.15,16 At least 30% of our cohort met eligibility criteria, but less than 10% underwent stress testing, including MPS and cardiovascular magnetic resonance imaging. We recognise that our reliance on ICA is because of an underdeveloped CTCA service, rather than greater perceived benefits of an invasive strategy. Despite this seeming compromise, the ICA service has reassuringly still delivered timely, safe diagnostic information for patients and clinicians. In figure 3 we have summarised the management of patients with chest pain of suspected cardiac origin according to national, international guidelines and local practice.
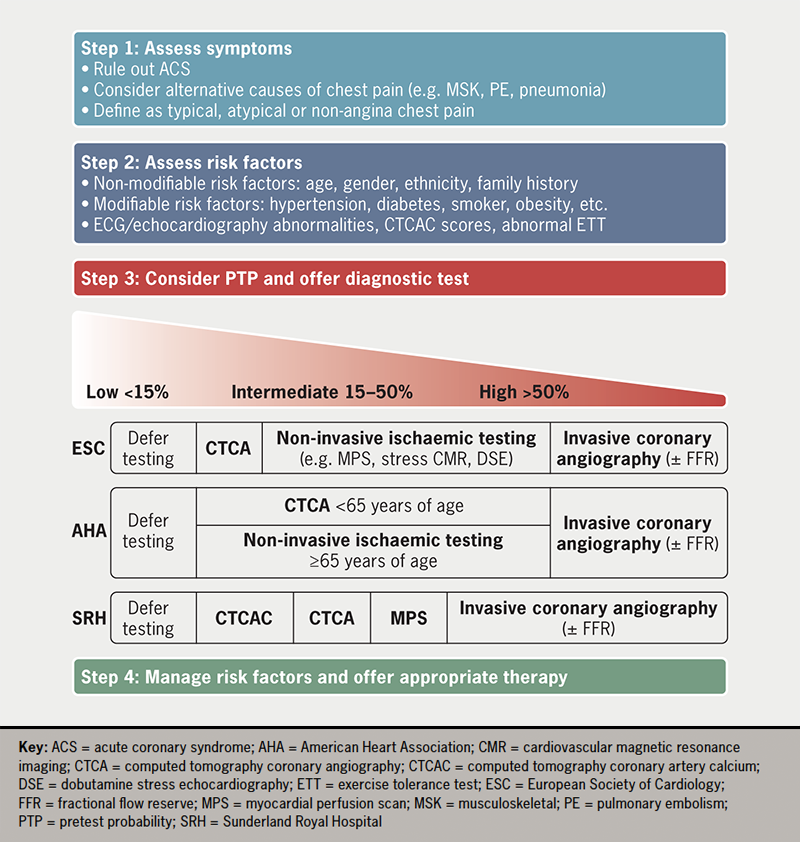
Our observations lend weight to the recommendations made in the Getting It Right First Time (GIRFT) review of cardiology in 2021, and the goals set out in NHS Long Term Plan, published in 2019.17–19 Both reports identified significant regional variation in access to and quality of cardiac care, particularly CTCA in the ‘stable chest pain pathway’.17–19 Important next steps in the delivery of a more equitable national CTCA service are a more than 100% increase in CT-scanning capacity and a further 2,000 radiologists and/or cardiologists accredited in CTCA reporting.19 Our data show that a full capacity CTCA service may avoid 75% of elective invasive coronary angiograms. Local PCI and complex device services could then be expanded, reducing the workload on tertiary centres as their volume of transcutaneous aortic valve implantations (TAVI) and other structural heart interventions, complex device extractions and ventricular tachycardia (VT) ablations increases. Ultimately, this would facilitate the development of a regional cardiology network, dictated by function and not geography, in Sunderland and the Northeast.18
Finally, this study has the inherent limitations of a retrospective analysis of clinical practice during a virus pandemic. We contend that the impact was small and that the data reflected current practice.
Conclusion
Our service evaluation of a RACPC showed that typical angina patients had a significantly higher likelihood of underlying obstructive CAD with increased risk of MACE than other chest pain patients. Despite NICE recommendations, a limited CTCA service led to a greater volume of ICA, which reassuringly still facilitated accurate diagnosis with the option of immediate revascularisation, timely and safely, in the right patients. However, a stricter referral triage system, as well as investment in and expansion of non-invasive anatomical and functional imaging capacity, may improve RACPC service delivery, support the larger cardiology network in the Northeast and, most importantly, offer safer and more equitable patient care.
Key messages
- In a major paradigm shift away from the modified Diamond-Forrester pre-test probability model, the National Institute for Health and Care Excellence (NICE), in 2016, recommended first-line computed tomography coronary angiography (CTCA) for patients presenting with chest pain of suspected cardiac origin
- Our service evaluation of a rapid access chest pain clinic (RACPC) provides valuable contemporary insights into local challenges, alternative management strategies and their impact on patient care and outcomes
- Our study emphasises the importance of considering other equally safe and effective anatomical and functional imaging techniques as CTCA service capacity continues to expand nationally
Conflicts of interest
None declared.
Funding
None.
Study approval
Ethical approval was waived as anonymised retrospective data were used.
Acknowledgement
We thank the staff at SRH for their high standard of care of the patients included in the study.
References
1. Skinner JS, Smeeth L, Kendall JM, Adams PC, Timmis A, Chest Pain Guideline Development Group. NICE guidance. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. Heart 2010;96:974–8. https://doi.org/10.1136/hrt.2009.190066
2. National Health Service. Coronary heart disease. National service framework for coronary heart disease. London: Department of Health, 2000. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/198931/National_Service_Framework_for_Coronary_Heart_Disease.pdf
3. National Institute for Health and Care Excellence. Recent-onset chest pain of suspected cardiac origin: assessment and diagnosis. London: NICE, 2016. Available from: https://www.nice.org.uk/guidance/cg95/evidence/full-guideline-pdf-245282221
4. SCOT-HEART Investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383–91. https://doi.org/10.1016/S0140-6736(15)60291-4
5. Dreisbach JG, Nicol ED, Roobottom CA, Padley S, Roditi G. Challenges in delivering computed tomography coronary angiography as the first-line test for stable chest pain. Heart 2018;104:921–7. https://doi.org/10.1136/heartjnl-2017-311846
6. Neumann FJ, Sousa-Uva M, Ahlsson A et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. https://doi.org/10.1093/eurheartj/ehy394
7. Thygesen K, Alpert JS, Jaffe AS et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 2018;72:2231–64. https://doi.org/10.1016/j.jacc.2018.08.1038
8. Douglas PS, Hoffmann U, Patel MR et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372:1291–300. https://doi.org/10.1056/NEJMoa1415516
9. Jordan KP, Timmis A, Croft P et al. Prognosis of undiagnosed chest pain: linked electronic health record cohort study. BMJ 2017;357:j1194. https://doi.org/10.1136/bmj.j1194
10. Sekhri N, Feder GS, Junghans C, Hemingway H, Timmis AD. How effective are rapid access chest pain clinics? Prognosis of incident angina and non-cardiac chest pain in 8762 consecutive patients. Heart 2007;93:458–63. https://doi.org/10.1136/hrt.2006.090894
11. Taylor GL, Murphy NF, Berry C et al. Long-term outcome of low-risk patients attending a rapid-assessment chest pain clinic. Heart 2008;94:628–32. https://doi.org/10.1136/hrt.2007.125344
12. Newby DE, Adamson PD, Berry C et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 2018;379:924–33. https://doi.org/10.1056/NEJMoa1805971
13. Otsuka K, Fukuda S, Tanaka A et al. Prognosis of vulnerable plaque on computed tomographic coronary angiography with normal myocardial perfusion image. Eur Heart J Cardiovasc Imaging 2014;15:332–40. https://doi.org/10.1093/ehjci/jet232
14. Royal College of Radiologists. Standards of practice of computed tomography coronary angiography (CTCA) in adult patients. London: Royal College of Radiologists, 2014. Available from: https://www.rcr.ac.uk/system/files/publication/field_publication_files/BFCR14%2816%29_CTCA.pdf
15. Gulati M, Levy PD, Mukherjee D et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021;144:e368–e454. https://doi.org/10.1161/CIR.0000000000001029
16. Knuuti J, Wijns W, Saraste A et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–77. https://doi.org/10.1093/eurheartj/ehz425
17. National Health Service. The NHS long term plan. London: Department of Health, 2019. Available from: https://www.longtermplan.nhs.uk/wp-content/uploads/2019/08/nhs-long-term-plan-version-1.2.pdf
18. Clarke S, Ray S. Cardiology: GIRFT programme national specialty report. London: GIRFT, 2021. Available from: https://www.gettingitrightfirsttime.co.uk/wp-content/uploads/2021/09/Cardiology-Jul21k-NEW.pdf
19. Richards M. Diagnostics: recovery and renewal. Report of the independent review of diagnostic services for NHS England. London: NHS England, 2020. Available from: https://www.england.nhs.uk/wp-content/uploads/2020/11/diagnostics-recovery-and-renewal-independent-review-of-diagnostic-services-for-nhs-england-2.pdf
