From the days of Virchow and the analysis of post-mortem coronary specimens, an enormous amount of knowledge has been built about coronary pathophysiology. In the 1950s the dream of in vivo coronary imaging became a reality with the invention of coronary arteriography under the guidance of Mason Sones. As we fast forward 50 years, it has become clear that angiography has helped us focus on areas of stenosis and flow limitation, but the main problem of coronary artery disease is much more complex than can appear on a luminal silhouette. The finding of ‘normal coronary arteries’ following angiography is short-sighted and does not take into account the potential of unstable disease lurking within the vessel wall. We begin the series with intravascular ultrasound.
Background
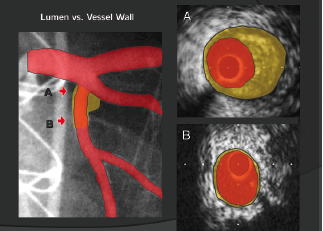
Coronary luminal narrowing is prevented by a vascular mechanism known as positive remodelling. This is an outwards expansion of the blood vessel to accommodate the build up of plaque within the artery (figure 1). This phenomenon appears within diseased segments of coronary arteries and the build up of these plaques is dependent on multiple, well-known factors from genetics and lifestyle through to coronary anatomy and flow patterns. The complex pathobiology that creates these plaques and leads to plaque structure weakening is beyond the scope of this review. However, contemporary coronary imaging can now focus on the vessel wall and identify high-risk, positively remodelled, plaques and their contents. This series of review articles will focus on what is now possible in the field of coronary artery imaging and how each modality can try to improve both the invasive treatment of unstable coronary syndromes and the prevention of unstable coronary artery disease in the future.
IVUS and virtual histology
Intravascular ultrasound (IVUS) is performed at the time of coronary angiography and involves a tiny ultrasound probe that emits high frequency signals (20–40 mHz). This wire-based probe can be placed over a coronary guidewire into the artery and withdrawn at a set rate (0.5 mm/sec) to provide segmental tomographic images of the vessel. IVUS has demonstrated discrepancies between the extent of atherosclerosis seen by coronary angiography and the actual extent of atherosclerotic disease.1 Quantitative assessment of the vessel and plaque within a lesion was made possible with the introduction of greyscale IVUS analysis and the further analysis of individual plaque components is now possible with virtual histology (VH).2
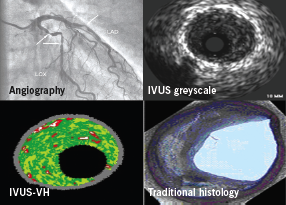
IVUS-VH uses advanced radiofrequency analysis of ultrasound backscatter signals to overcome the limitations of greyscale IVUS by providing a more detailed analysis of plaque morphology (figure 2).3 In addition, IVUS-VH has the potential to provide in vivo patient-specific plaque analysis to determine the range of characteristics in relation to clinical factors and risk, rather than making assumptions about living plaque from a highly selected autopsy population.
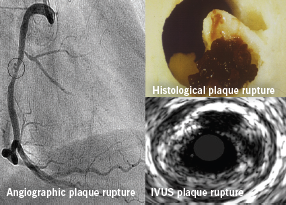
Greyscale IVUS has also demonstrated the multiplicity of plaque ruptures seen in patients with acute coronary syndromes (ACS) (figure 3).4-6 A recent study also highlighted that the number of vulnerable plaques with less than 75% luminal obstruction identified by IVUS had a positive correlation with future cardiovascular events.7 Of note, serial IVUS analysis of a small patient cohort showed that 50% of ruptured coronary plaques detected on first ACS event had spontaneously healed at 22 months’ follow-up.8
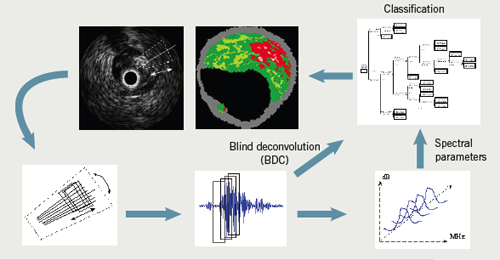
Obviously, many different cell and tissue types are commonly found in atherosclerotic plaques. To simplify image interpretation, and because of the fundamental resolution limitations of the underlying ultrasound signal, plaque components are grouped into four basic tissue types during IVUS-VH imaging. These components are displayed on the image as different colour pixels. This technique is based on advanced radiofrequency analysis of reflected ultrasound signals in a frequency domain analysis and displays the reconstructed colour-coded tissue map of plaque composition superimposed on cross-sectional images of the coronary artery obtained by greyscale IVUS (figure 4).3
Tissue types
1. Fibrous
Fibrous tissue is represented by dark-green pixels (figure 5). Histologically, this tissue is collagenous with no lipid.9,10 On greyscale IVUS, these tissues tend to be medium-bright regions.
2. Fibrofatty
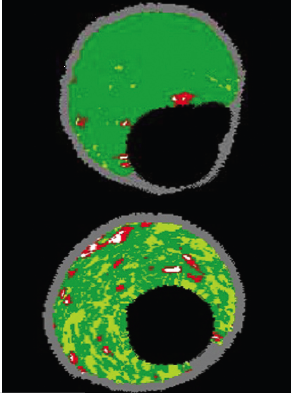
Fibrofatty tissue is denoted in VH by light-green pixels (figure 5). This tissue is loosely packed collagen, but it can have a cellular quality with potential for foam cells to start invading.10 There is usually no necrotic core and even cholesterol products are rare. If thrombus or plaque rupture are included as plaque during analysis, then they are displayed as fibrofatty plaque.11
3. Necrotic core
In VH the necrotic core is seen as red (figure 6). This tissue is a mixture of soft, lipid-like dead cells, foam cells and trapped blood cells.9 Most of any real structure is lost and with some areas producing micro-calcification as a by-product (from the dead cells) this leads to a recipe for gross instability and rupture with friable areas next to sharp calcification.
4. Dense calcium
White pixels represent dense calcium (figure 6). These calcified regions can be lost during histology processing but on plain greyscale IVUS, they act as extremely strong reflectors of signal and appear as bright white.
Plaque risk assessment

In vivo plaque classification with IVUS-VH is based on a histopathological classification system developed by Virmani et al. in 2000.12,13 From this system, coronary lesions can be classified as adaptive intimal thickening, pathologic intimal thickening, fibroatheroma, fibrocalcific and thin-cap fibroatheroma (TCFA) plaques. For the purpose of this review we focus on the highest-risk plaque type described below.
Thin-cap fibroatheroma (TCFA)
TCFA have a confluent necrotic core (>10%) in direct contact with the lumen (no IVUS evidence of a cap) and a minor amount of calcium (<10%). If this is present on three consecutive IVUS-VH cross-sectional frames, this confers an increase in vulnerability. It is currently thought that the higher the extent of surface contact the necrotic core has with the lumen, and the presence of increased amounts of calcium, produces the highest risk of rupture. This appears to be in some way different from the commonly taught theory that non-significant plaques are the ones which are more likely to rupture.14 It may simply be that non-significant plaques are just more frequent along a given coronary segment.
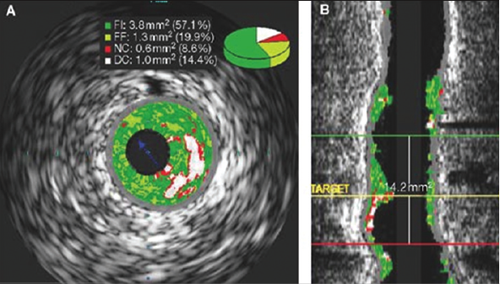
As the majority of acute coronary events are triggered by plaque rupture,4,5 defining the anatomic features that lead to plaque rupture should be of central importance to lesion imaging. Post-mortem analyses have shown that TCFA is probably the main precursor lesion for plaque rupture.12 According to histological studies, the size of the necrotic core and the thickness of the fibrous cap have a critical influence on plaque stability, along with our previously mentioned positive remodelling. In addition, other characteristics of vulnerable lesions such as micro-calcification within the lesion, its location within the coronary tree, the length of the lesion and the degree of stenosis relative to healthy reference lumen size, are also important parameters for the evaluation of plaque vulnerability. The reconstruction of IVUS-VH images in a longitudinal view enables a comprehensive analysis of the total length of the plaque and its complete orientation to the rest of the coronary tree (figure 7).
Therefore, exact coronary dimensions and elements of plaque composition, such as the presence and amount of necrotic core, plaque burden, the degree of calcification and coronary remodelling, are all anatomic features visualised by IVUS and IVUS-VH, but not by traditional angiography. However, the limited resolution of IVUS-VH (approximately 150 µm) renders it unable to truly claim the ability to visualise thin fibrous caps (<65 µm). In order to improve visualisation of the coronary lumen we need to improve resolution, and this is best seen with a newer invasive tool called optical coherence tomography (OCT). OCT uses spectroscopic light emission in place of the ultrasound waves and will be covered in our next article.
Summary
Angiographic imaging of coronary disease is flawed and cannot be relied on to understand the full extent of plaque development. Imaging tools are now available that can directly image the vessel wall and calculate plaque burden and plaque composition with great accuracy. IVUS remains an under-utilised but invaluable clinical and research tool to improve the understanding of coronary artery disease. It is hoped that in the future it may not only help improve the outcome from vulnerable plaque treatment by percutaneous intervention,
but also help stratify the vulnerable patient to improve risk factor modification and appropriate preventative drug therapy.
Conflict of interest
None declared
Key messages
- Angiographic assessment of coronary plaque is limited and does not provide the full story
- Intravascular ultrasound (IVUS) can image the entire vessel in tomographic slices around 0.01 mm thick and with 150 µm resolution
- Virtual histology (VH) is a new technique that can provide a sensitive guide to plaque components within four groups: fibrous, fibrofatty, necrotic core, and dense calcium
- It is hoped that future studies will show that IVUS-VH can improve results and outcomes from coronary intervention
References
1. Nissen SE, Yock P. Intravascular ultrasound: novel pathophysiological insights and current clinical applications. Circulation 2001;103:604–16.
2. Nissen SE. Application of intravascular ultrasound to characterize coronary artery disease and assess the progression or regression of atherosclerosis. Am J Cardiol 2002;89:24B–31B.
3. Nair A. Coronary plaque classification with intravascular radiofrequency data analysis. Circulation 2006;106:2200–06.
4. Tanaka A, Shimada K, Sano T et al. Multiple plaque rupture and C-reactive protein in acute myocardial infarction. J Am Coll Cardiol 2005;45:1594–9.
5. Rioufol G, Finet G, Ginon I et al. Multiple atherosclerotic plaque rupture in acute coronary syndrome: a three-vessel intravascular ultrasound study. Circulation 2002;106:804–08.
6. Schoenhagen P, Stone GW, Nissen SE et al. Coronary plaque morphology and frequency of ulceration distant from culprit lesions in patients with unstable and stable presentation. Arterioscler Thromb Vasc Biol 2003;23:1895–900.
7. Yamagishi M, Terashima M, Awano K et al. Morphology of vulnerable plaque: insights from follow-up of patients examined by intravascular ultrasound before an acute coronary syndrome. J Am Coll Cardiol 2000;35:106–11.
8. Rioufol G, Finet G, Ginon I et al. Evolution of spontaneous atherosclerotic plaque rupture with medical therapy: long-term follow-up with intravascular ultrasound. Circulation 2004;110:2875–80.
9. Burke AP, Kolodgie FD, Farb A et al. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation 2002;105:297–303.
10. Nair A, Margolis MP, Kuban VD, Vice DG. Automated coronary plaque characterisation with intravascular ultrasound backscatter: ex vivo validation. Eurointervention 2007;3:113–20.
11. Murray SW, Palmer ND. Intravascular ultrasound and virtual histology interpretation of plaque rupture and thrombus in acute coronary syndromes. Heart 2009;95:1494.
12. Kolodgie FD, Virmani R, Burke AP et al. Pathologic assessment of the vulnerable human coronary plaque. Heart 2004;90:1385–91.
13. Uemura R, Tanabe J, Ohaki M et al. Impact of histological plaque characteristics on intravascular ultrasound parameters at culprit lesions in coronary artery disease. Int Heart J 2006;47:683–92.
14. Kolodgie FD, Gold HK, Burke AP et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 2003;349:2316–25.
