In recent years a large amount of research has focused on developing both invasive and non-invasive methods of assessing atherosclerosis. In this regard, magnetic resonance imaging (MRI) is safe, non-invasive, requires no ionising radiation, and is capable of giving high-resolution images of atherosclerotic plaque. As a result, MRI has been extensively applied to imaging of the vascular system – in particular, the carotid arteries – where it has been shown to have the ability to not only accurately quantify the extent of atherosclerotic plaque disease, but also to identify several compositional features suggestive of plaque vulnerability. Imaging of the relatively small coronary arteries has, until now, been limited by the problems of cardiac and respiratory motion, however, more recently, technological advancements have allowed more detailed plaque information to be acquired. This article will review the origins of MRI imaging of atherosclerotic disease, its current status, and its potential future applications.
Background: carotid/vascular MRI
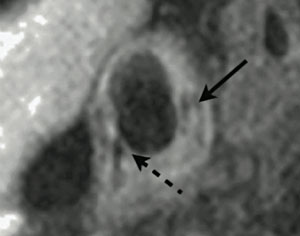
Magnetic resonance arteriography (MRA) has for many years been used as a non-invasive means of producing an arterial lumenogram, an image of flow down the arterial lumen, from which the presence of significant stenosis could often be detected, if needed, by comparison to the comparatively normal flow in the contralateral vessel. However, the first description of the use of magnetic resonance imaging (MRI) to describe direct imaging of atherosclerotic plaque itself was by Gold in 1993.1 The authors used ex vivo human aorta specimens containing atheroma to correlate MRI signal changes with the presence of specific histological features, including lipid-rich necrotic core, calcification, and fibrous plaque. Following this, the first in vivo description of MRI plaque imaging was performed in 1996 on six patients prior to carotid endarterectomy.2 Histological verification of these images with the carotid endarterectomy specimens clarified that MRI was capable of distinguishing a number of plaque features, including lipid cores, fibrous caps, calcification, haemorrhage and acute thrombosis, in addition to the normal media and adventitia of the vessel wall (figure 1). This discovery stimulated a large amount of subsequent research into carotid plaque MRI,3-6 ultimately leading to a modification of the American Heart Association (AHA) plaque classification system to allow MRI grading of carotid atherosclerotic plaque.7 More recent work has begun to move towards potential clinical uses of carotid MRI imaging. For example, Yuan et al. have described the positive association between identification of a ruptured fibrous cap on MRI, and a recent history of transient ischaemic attack (TIA) or stroke.3 In prospective studies, the presence of intraplaque haemorrhage on carotid MRI has been shown to predict the short-term combined risk of ipsilateral TIA and stroke,8 and, in addition, the chances of cerebral embolisation during surgery.8 Although larger long-term clinical studies are required, these studies indicate that carotid plaque MRI may have an important future role in the assessment and treatment of TIA and stroke.
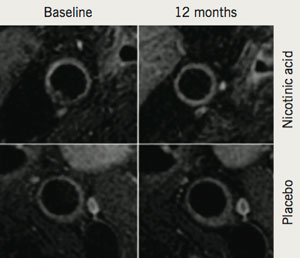
In addition to its potential clinical applications, the ability of MRI to provide highly accurate information on plaque burden and morphology has been used to examine the effect of both established and novel pharmacotherapies on the vasculature in the research and clinical trial domains. Corti et al. were the first to demonstrate a reduction in carotid and aortic atherosclerosis using serial MRI after 12 months of statin treatment.9 Subsequently, it was demonstrated that more intensive lipid lowering, to low-density lipoprotein (LDL)-cholesterol <100 mg/dL, was associated with a larger decrease in plaque size, also over 12 months.10 MRI has also been used to provide further mechanistic insights into how atherosclerotic plaque responds to cholesterol-altering medications. Lee et al. used MRI to demonstrate, in the carotid arteries and aorta, reduction in the plaque index (normalised vessel wall area) as early as three months after statin initiation. In the same patients, early changes in atherosclerosis (within three months) were significantly correlated with later change at 12 months.11 More recently, MRI has been used to investigate the effects of various treatment strategies, including the use of niacin to increase levels of high-density lipoprotein (HDL) (figure 2).12 As a result of these successes, MRI of atherosclerotic plaque is currently being used in phase III trials of novel therapeutic agents.
Coronary MRI
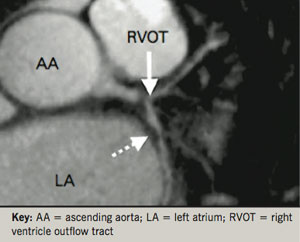
MRI of the coronary arteries has progressed relatively more slowly. Not only are the coronary arteries small, but they are in constant motion and have similar characteristics to the surrounding myocardium and cardiac veins. Nonetheless, MRA of the coronary arteries, allowing the detection of stenotic plaque disease, has now been performed for over a decade, and was originally shown to be able to reliably detect patients with left main coronary or three-vessel disease (figure 3).13 Subsequently, in an attempt to improve acquisition times, whole heart magnetic resonance (MR) coronary angiography has been developed.14 This allows free-breathing images to be taken during a patient-specific time window of the cardiac cycle during which coronary artery motion is minimal. In a manner analogous to coronary computed tomography (CT), the upper and lower boundaries of the heart are defined in order to allow imaging over a single volume that covers both coronary arteries. As a result, images are simpler to acquire, and the scan can be performed in a much shorter time period. Using this technique, Sakuma et al. demonstrated that significant narrowing of coronary arterial segments with a diameter >2 mm could be detected with moderate sensitivity (82%) and high specificity (90%).15 However, despite technological advances such as this, the overall acquisition speed of 3D MRA remains considerably slower (>2 minutes) than that of multi-slice CT (<2 seconds). Nonetheless, it should be noted that MR coronary imaging does hold several advantages over CT plaque imaging: the ability to safely perform serial imaging, the lack of need for an injected contrast agent in image acquisition, and the ability to characterise heavily calcified areas of the coronary tree.16 Currently, however, clinical guidelines only recommend the use of coronary MRA for determining the proximal course of anomalous coronary arteries.

More recent work has suggested a brighter future for MRI imaging of coronary atherosclerotic plaque. In addition to coronary MRA, black-blood imaging of the coronary artery wall is now possible, which allows detection of increased wall thickness in patients with angiographically documented coronary artery disease with high reproducibility.17,18 In a recent sub-study of the Multi-Ethnic Study of Atherosclerosis (MESA) trial, 179 asymptomatic patients with subclinical atherosclerosis underwent coronary wall MRI.19 Although no significant coronary artery narrowing was detected by MRA, direct wall imaging noted that as the arterial wall became thicker, the luminal diameter remained relatively constant; in contrast, the outer vessel wall was seen to expand with the thickened arterial wall. This phenomenon, termed “positive remodelling” and first described by Glagov,20 highlights the need to develop accurate methods of direct coronary plaque imaging using MRI, in a similar manner to those methods currently used for carotid plaque. Currently, non-contrast T1-weighted imaging of coronary plaque can identify high-intensity signal (HIP) (figure 4), which has recently been associated with positive coronary remodelling and low CT signal density.21 However, the prognostic significance of HIP lesions is currently unknown.22 Compared with carotid imaging, MRI of coronary atherosclerotic plaque is currently unable to identify individual plaque features with the same sensitivity and specificity.
Future directions
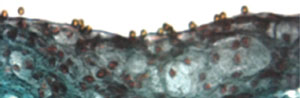
Ongoing improvements in imaging sequences, combined with higher field strength imaging, are likely to enhance the ability of MRI to provide high-resolution plaque images while simultaneously decreasing scan acquisition times. In addition, several novel contrast agents are under investigation that may permit MRI to simultaneously provide information on both vascular biology and morphology. For example, McAteer et al. constructed a dual-ligand microparticle of iron-oxide (4.5 µm diameter) to target endothelial P-selectin and vascular cell adhesion molecule (VCAM)-1 (figure 5).23 Binding of the microparticles to inflamed mouse endothelium was subsequently shown by high resolution ex vivo MRI (9.4 T). In human work, Tang et al. have demonstrated the use of ultrasmall particles of iron oxide (USPIO) to provide information on carotid plaque macrophage content using 1.5 T MRI.24
Conclusions
MRI is emerging as a leading non-invasive modality for the assessment of atherosclerotic plaque disease. While MRI of atherosclerotic plaque in the coronary arteries awaits further technical developments, imaging of plaque in the carotid arteries has already shown that MRI is capable of giving detailed information both on vessel wall thickness and plaque composition. With the ongoing development of novel contrast agents, MRI is likely to play a major role in the future development of imaging strategies to aid risk stratification and treatment in ischaemic vascular disease.
Conflict of interest
None declared.
Key messages
- Magnetic resonance imaging (MRI) is one of the leading non-invasive plaque imaging modalities
- Detailed images are available for carotid artery plaque and there is excellent promise for tissue characterisation
- The absence of radiation exposure permits serial studies of plaque over time
- There is the potential for a “one-stop shop” able to provide every facet of cardiovascular imaging in one scan
- Routine coronary imaging will require further improvements in technology and resolution
References
- Gold GE, Pauly JM, Glover GH et al. Characterization of atherosclerosis with a 1.5-T imaging system. J Magn Reson Imaging 1993;3:399–407.
- Toussaint JF, LaMuraglia GM, Southern JF et al. Magnetic resonance images lipid, fibrous, calcified, hemorrhagic, and thrombotic components of human atherosclerosis in vivo. Circulation 1996;94:932–8.
- Yuan C, Zhang S-X, Polissar NL et al. Identification of fibrous cap rupture with magnetic resonance imaging is highly associated with recent transient ischemic attack or stroke. Circulation 2002;105:181–5.
- Yuan C, Mitsumori LM, Ferguson MS et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation 2001;104:2051–6.
- Shinnar M, Fallon J, Wehrli S et al. The diagnostic accuracy of ex vivo MRI for human atherosclerotic plaque characterization. Arterioscler Thromb Vasc Biol 1999;19:2756.
- Hatsukami TS, Ross R, Polissar NL, Yuan C. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation 2000;102:959–64.
- Cai J-M, Hatsukami TS, Ferguson MS et al. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002;106:1368–73.
- Altaf N, MacSweeney ST, Gladman J, Auer DP. Carotid intraplaque hemorrhage predicts recurrent symptoms in patients with high-grade carotid stenosis. Stroke 2007;38:1633–5.
- Corti R, Fayad Z, Fuster V et al. Effects of lipid-lowering by simvastatin on human atherosclerotic lesions: a longitudinal study by high-resolution, noninvasive magnetic resonance imaging. Circulation 2001;104:249–52.
- Corti R, Fuster V, Fayad ZA et al. Lipid lowering by simvastatin induces regression of human atherosclerotic lesions: two years” follow-up by high-resolution noninvasive magnetic resonance imaging. Circulation 2002;106:2884.
- Lee JMS, Wiesmann F, Shirodaria C et al. Early changes in arterial structure and function following statin initiation: quantification by magnetic resonance imaging. Atherosclerosis 2008;197:951–8.
- . Lee JM, Robson MD, Yu LM et al. Effects of high-dose modified-release nicotinic acid on atherosclerosis and vascular function: a randomized, placebo-controlled, magnetic resonance imaging study. J Am Coll Cardiol 2009;54:1787–94.
- . Kim W, Danias P, Stuber M et al. Coronary magnetic resonance angiography for the detection of coronary stenoses. N Engl J Med 2001;345:1863.
- . Stehning C, Boernert P, Nehrke K. Advances in coronary MRA from vessel wall to whole heart imaging. Magn Reson Med Sci 2007;6:157–70.
- Sakuma H, Ichikawa Y, Chino S et al. Detection of coronary artery stenosis with whole-heart coronary magnetic resonance angiography. J Am Coll Cardiol 2006;48:1946–50.
- . Liu X, Zhao X, Huang J et al. Comparison of 3D free-breathing coronary MR angiography and 64-MDCT angiography for detection of coronary stenosis in patients with high calcium scores. AJR Am J Roentgenol 2007;189:1326–32.
- Fayad ZA, Fuster V, Fallon JT et al. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation 2000;102:506–10.
- Botnar RM, Perez AS, Witte S et al. In vivo molecular imaging of acute and subacute thrombosis using a fibrin-binding magnetic resonance imaging contrast agent. Circulation 2004;109:2023–9.
- Miao C, Chen S, Macedo R et al. Positive remodeling of the coronary arteries detected by magnetic resonance imaging in an asymptomatic population: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol 2009;53:1708–15.
- Glagov S, Weisenberg E, Zarins C et al. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 1987;316:1371–5.
- Kawasaki T, Koga S, Koga N et al. Characterization of hyperintense plaque with noncontrast T(1)-weighted cardiac magnetic resonance coronary plaque imaging: comparison with multislice computed tomography and intravascular ultrasound. JACC Cardiovasc Imaging 2009;2:720–8.
- Tanaka A, Kawasaki T, Noguchi T et al. Hyperintense plaque with noncontrast t1-weighted magnetic resonance coronary plaque imaging leading to acute coronary syndrome. Circulation 2009;120:2400–01.
- McAteer MA, Schneider JE, Ali ZA et al. Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler Thromb 2007;28:77–83.
- Tang TY, Howarth SP, Miller SR et al. The ATHEROMA (atorvastatin therapy: effects on reduction of macrophage activity) study: evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol 2009;53:2039–50.
