The amyloidoses comprise a collection of disorders in which proteins, some native and some mutated, are deposited in tissues. These proteins self-assemble themselves to form an ordered fibrillar matrix termed amyloid. Currently, more than 20 different proteins have been identified, the most common with as many as 100 different mutations per protein. Despite these figures, the conditions that arise clinically are not that common. This undoubtedly results in a number of such individuals not being identified, or typically only when it is too late to effect a cure.
This article describes the features, diagnosis and treatments for the different types of amyloid that affect the heart.
Introduction
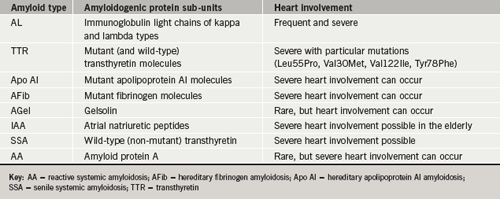
Several types of amyloidosis involve the heart (table 1), often with devastating consequences. In many cases prognosis is further worsened by systemic multi-organ involvement. For the primary care physician, two types are important to identify as they will often present with heart involvement. These include AL amyloid (previously termed primary) and some of the hereditary forms of amyloid.
The remaining forms either involve the heart rarely, as in secondary amyloid (AA) or rather late in life as in cases of senile systemic amyloid (SSA) and isolated atrial amyloid (IAA). Notwithstanding, these latter forms are still capable of causing significant mechanical dysfunction and rhythm disturbance.
Making a diagnosis
Many disorders can be confused with amyloid heart disease, most commonly forms of intrinsic hypertrophic cardiomyopathy (HCM), other deposition diseases and in some cases Fabry’s disease.1
Cardiac involvement is usually first suspected from the appearances on echocardiography. The heart walls are usually globally thickened and characteristically this often includes the inter-atrial septum. The myocardium demonstrates an increased scintillation pattern (granular sparkling) although this is certainly not specific for amyloid. Valve leaflets and the pericardium may be thickened and there is often a modest pericardial effusion. While both atria will usually be dilated, the ventricular chambers are rarely dilated. Doppler interrogation of ventricular inflow will show the characteristic restrictive features of an infiltrative cardiomyopathy.
A low or normal voltage electrocardiogram (ECG) in the presence of apparent left ventricular ‘hypertrophy’ is very suggestive of heart involvement, as are Q-waves without prior history of myocardial infarction. Tissue Doppler techniques have proved helpful in distinguishing amyloid from other causes of true hypertrophy and in characterising ventricular dysfunction.2 Myocardial velocity profiles in patients with amyloid heart involvement show characteristics that differ from hypertrophied ventricular walls due to hypertension or HCM. Imaging amyloid heart disease using magnetic resonance imaging (MRI) with gadolinium enhancement, can also help differentiate amyloid from other causes of wall thickening, particularly HCM.3
Serum amyloid P scintigraphy scanning
The extent of amyloid deposition, of any type, can be assessed by serum amyloid P component (SAP) scintigraphy.4 SAP is a normal plasma protein that binds reversibly to amyloid deposits of any type. While useful for imaging and quantifying amyloid in the liver, kidneys, spleen and bone marrow, it is not useful for identifying amyloid in the heart, due to the large blood pool and cardiac motion.
Histological and genetic diagnostic procedures
A formal histological diagnosis is made from either a screening biopsy, such as rectal or abdominal fat biopsy, or from an affected organ, for example kidney, liver or nerve. Amyloid deposits stain with Congo red and produce red–green birefringence when viewed under cross-polarised light. Cardiac biopsy may be indicated when there is no suggestion of extra-cardiac amyloid or when the diagnosis is uncertain. In AL amyloid, serum and or urine electrophoresis and immunofixation will detect a monoclonal immunoglobulin in 80–90% of cases. The availability of techniques to estimate free light chains has revolutionised the diagnosis and management of AL amyloid disease. In cases where there remains doubt about amyloid fibril type, DNA analysis to exclude hereditary amyloidosis and even amyloid fibril protein sequencing may be required. A bone marrow biopsy should be performed to quantify the plasma cell population, exclude myeloma and also to stain for amyloid, which if present is strongly suggestive of AL type.
AL amyloidosis

AL amyloidosis is the most commonly recognised form of amyloid in the UK. AL amyloid fibrils are derived from monoclonal immunoglobulin light chains produced by a plasma cell dyscrasia. While organ dysfunction is probably due, in the main, to the disruptive physical presence of amyloid deposits, there is good evidence that these light chains are inherently cytotoxic.5,6
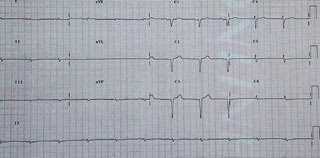
AL amyloid presenting with heart failure has a very poor prognosis, with a median survival reported as low as four months.7 In the absence of treatment, the natural history of AL amyloidosis is that of a progressive and fatal disease within two years in about 80% of patients.8 The disease is usually renal or cardiac dominant in character with death an early outcome in the absence of treatment.7,9Table 2 illustrates the typical clinical features found in AL amyloid disease. A low or normal voltage ECG (figure 1) in the presence of apparent left ventricular ‘hypertrophy’ on echo (figure 2) is very suggestive of AL heart involvement, as are Q-waves without prior history of myocardial infarction. Additional electrocardiographic and echo features of AL amyloid heart involvement are shown in table 3 and figure 2.

Management of amyloid heart disease
Patients with AL (and non-AL) forms of cardiac involvement may benefit symptomatically from conventional heart failure therapies. Careful titration of diuretics remains the mainstay of management. Orthostatic hypotension may require support stockings and sometimes the use of fludrocortisone. The alpha agonist midodrine can be very effective as a pressor agent. Calcium channel blockers and beta blockers should be used with caution due to their negative inotropic effects. Reports suggest digoxin can bind to amyloid fibrils with increased toxicity, but judicious low-dose treatment can sometimes be beneficial. Permanent pacing, resynchronisation pacing and automatic implantable cardioverter defibrillator (AICD) implantation may prove useful in a small proportion of cases.
Specific management of AL amyloid
Treatment of AL amyloidosis comprises chemotherapy directed towards the underlying clonal plasma cell disease, with the objective of reducing production of amyloidogenic monoclonal immunoglobulin light chains. Combined oral melphalan and prednisolone is of proven but very modest benefit in AL amyloidosis10 and more dose-intensive regimens are now usually pursued. Recent British guidelines favour intermediate-dose chemotherapies, such as vincristine, adriamycin and dexamethasone (VAD) or monthly intravenous melphalan with dexamethasone.11
Chemotherapy that reduces circulating free immunoglobulin light chain (FLC) concentrations can greatly enhance survival.12,13 In a recent study, the five-year survival of AL patients was 88% in those with more than a 50% reduction in their FLC, compared to 39% in those whose FLC did not fall by half (p<0.0001).13
High-dose chemotherapy and autologous stem cell transplantation
High-dose chemotherapy with melphalan supported by autologous stem cell transplantation has been used increasingly in AL amyloidosis.14 Response rates in terms of clonal disease remission are encouraging with centres reporting a complete haematologic response in as many as 40% of eligible patients. However, mortality rates are appreciable with experienced centres reporting values between 13% and 20%.15,16 The overall impression is that patients with advanced disease, and particularly those with cardiac decompensation, tolerate this therapy poorly.
Additional and alternative therapies
Following success with its use in myeloma, the drug thalidomide has been tried both alone and in combination with chemotherapy,17 although high-dose thalidomide is not well tolerated by subjects with AL amyloid.18 Recently, the combination of thalidomide and intermediate-dose dexamethasone has been shown to be effective in a proportion of patients (48%) who are refractory to therapy. Again, as with high-dose thalidomide, treatment-related toxicity was frequent (65%).19
The tumour necrosis factor inhibitor etanercept has been trialled in a small cohort with advanced AL amyloidosis, with 50% of patients appearing to benefit objectively and 88% reporting subjective benefit in symptoms.20

As yet there is no specific treatment for amyloid that has been proven to promote its regression, though many candidate drugs have been tested over the years. Recent studies of a doxorubicin derivative (I-DOX), a cytotoxic anthracyclin, suggested promise,21 but subsequent data were inconclusive,22,23 and it is notably toxic.
Heart transplantation for AL amyloid heart disease
The UK experience was reported in 2004 for a total of 24 patients (17 AL and seven non-AL amyloid).24 Regardless of the use of adjunctive chemotherapy, the five-year survival after heart transplantation was generally poorer for AL (20% at five years), but similar for non-AL amyloidosis (64% at five years), than following heart transplantation for other indications. Progression of the systemic disease contributed to the increased mortality. Experience from the United States is similarly disappointing in AL patients undergoing heart transplantation.25
The non-AL amyloidoses
Within the non-AL categories of amyloid, of the 75 mutations known to express a clinical phenotype, around 44 (59%) involve the heart with a cardiomyopathy. Some deposited proteins cause cardiac compromise on a par with AL amyloid. The true frequencies of these individual types of amyloid are very difficult to estimate, largely due to the fact that many patients remain undiagnosed or misdiagnosed, and it may be of relatively late onset in life.
In the non-AL types, inquiry about a family history, with particular attention to any neurological diseases, is important. The caveat to this is that penetrance of these autosomal dominant genes is not always 100%. Patients with many of the non-AL forms of inherited amyloidosis frequently present with a progressive sensorimotor neuropathy. Macroglossia is not a feature, although carpal tunnel syndrome may be an early indicator of the disease. Clinical features will depend on the protein sub-unit, the actual mutation and, frequently, the kindred within which the mutation is ‘embedded’.
As in AL amyloid heart disease, a restrictive cardiomyopathy is often the ultimate cause of death (figure 1), and fatal cardiac arrhythmias are also a feature. Echocardiographically, a hereditary form of amyloidosis may be indistinguishable from AL amyloidosis.26
Hereditary amyloidosis
Autosomal dominant hereditary amyloid is probably the second most common type of amyloid encountered by cardiologists, though the familial aetiology is often not obvious. Usually these forms are associated with a mutation of the plasma protein transthyretin (TTR). Around 100 different amyloidogenic mis-sense point mutations of TTR have already been described. One such is particularly common in patients of black African origin. Heart involvement is not uncommon and can also occur with variants of hereditary fibrinogen and apolipoprotein-AI amyloid.
Management of hereditary amyloidosis
In patients with hereditary amyloidosis, where the amyloidogenic protein is predominantly produced by the liver (TTR and fibrinogen mutations), orthotopic liver transplantation provides a treatment by removing the source of the mutant protein.27,28 Initial hopes for liver transplantation as a cure have been tempered by reports of progression of amyloid deposition in native hearts and donor hearts simultaneously transplanted with livers.29 It appears that wild-type TTR may continue the amyloid deposition, after liver transplantation has eliminated the TTR variant that initiated the amyloidogenic process.30
Experience with apolipoprotein AI amyloidosis and cardiac involvement is less well described. Patients with mutations of the apoprotein AI molecule, may require combined heart and kidney transplantation, because of a predilection for apoprotein AI amyloid deposition in the kidneys with resultant renal failure.
Additional and alternative therapies
A drug is already being tested in patients that targets SAP with the goal of eliminating SAP from amyloid deposits, in the hope that this may reduce amyloid deposition and/or accelerate amyloid clearance. Small molecule ligands that stabilise the native tetrameric structure of TTR and prevent its fibrillogenesis are being actively investigated for prophylaxis and therapy in TTR amyloidosis. Diflusinal has recently been found to stabilise the structure of TTR. This action reduces tetramer dissociation and subsequent monomer misfolding and aggregation into amyloid. A trial of its clinical efficacy is in progress and several similar, and possibly more potent, agents are in development.
Senile systemic amyloidosis
Normal wild-type TTR is in itself weakly amyloidogenic and wild-type TTR amyloid deposition is remarkably common in individuals over 70–80 years of age. Unlike genetically variant forms, wild-type TTR amyloid deposits are largely confined to the heart.31 Cardiac deposition of wild-type TTR may sometimes be massive, resulting in severe heart failure.32 The echocardiographic
appearance is typical of other forms of amyloidosis, but there is no neuropathy or other major extracardiac involvement. It is almost exclusively a disease of elderly men. A recent report comparing patients with AL and senile amyloid heart involvement found that patients with senile cardiac amyloid had ventricular walls even thicker than those with AL.33 However, despite thicker walls and being older, the senile amyloid patients had less severe heart failure and a much longer median survival (75 vs. 11 months). Treatment is directed at symptom relief with conventional heart failure therapies. Heart transplantation is a reasonable consideration in patients with advanced SSA heart involvement, but only if they present at a young enough age. Despite the terminology (‘senile’ amyloid), occasionally patients are under 60 years of age and eligible for transplantation.24
Secondary amyloidosis
AA amyloidosis is a rare complication of chronic inflammatory disorders. The fibrils are derived from the acute phase reactant serum amyloid A protein. Although cardiac deposits are often present at histology, echocardiographic abnormalities and clinical symptoms of cardiac AA amyloidosis are extremely rare, occurring in about 2% of cases. The prognosis is substantially better than in cases of AL amyloid.34 Treatment involves suppressing the underlying inflammatory disease.
Isolated atrial amyloid
Atrial natriuretic peptide (ANP) is synthesised locally by atrial myocytes,35 and can be deposited locally within the atria as amyloid. It may be important in the development of atrial conduction abnormalities and atrial fibrillation. IAA is a disease of the elderly, with a female preponderance that contrasts with an almost male exclusivity of senile TTR amyloid.33 The incidence of IAA in elderly hearts is high, with one autopsy study describing IAA in 91 of 100 hearts.36 No specific therapy exists to treat IAA and management centres on controlling rhythm disturbance.
Conclusion
Late diagnosis remains one of the main hindrances to the management of amyloidosis. Once identified, it is vital to precisely determine the type of amyloid as both the prognosis and treatment differ very considerably among the types.
The goal remains, to prevent production and deposition of constituent monomer building blocks, to destabilise assembled fibrils and solubilise the amyloid deposits into non-pathogenic constituents.
Acknowledgements
The author wishes to thank Professor Philip Hawkins, of the National Amyloid Unit, the Royal Free Hospital, London, for his help and advice in preparing this manuscript and Professor Rodney Falk, for his advice and information, from the Amyloid Treatment and Research Program, Boston University School of Medicine, Boston, Massachusetts, USA.
Conflict of interest
SWD has received payments from Pfizer for data analysis on drug development in the treatment of amyloid.
Key messages
Amyloid disease
- The National Amyloid Centre for the UK is located at the Royal Free Hospital and provides a diagnostic and management advisory service for the NHS
- The diagnosis is frequently missed and, when detected, the type of amyloid incorrectly assigned
- A signatory cardiac feature is a thick-walled heart in combination with a low-voltage electrocardiogram
- The amyloidoses are systemic diseases that may mimic many other clinical conditions
- The clinical importance of senile systemic amyloid (SSA) may be underestimated
- Always ask about a family history, particularly of neurological disease
AL amyloid
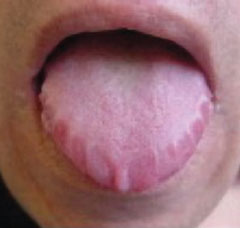
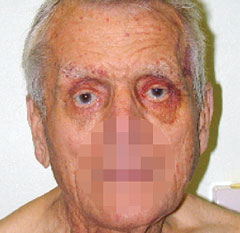
- Classic stigmata of AL amyloid include macroglossia and ‘raccoon eyes’
- Consider the diagnosis in patients with nephrotic range proteinuria and heart failure
- Consider occult involvement of other vital organs when haemodynamic status is impaired, i.e. thyroid and adrenal glands
- Advanced cardiac disease at presentation carries a very poor prognosis
- Isolated heart involvement is extremely unusual in this systemic disease
- Sudden death is common
References
- Morner S, Hellman U, Suhr OB, Kazzam E, Waldenstrom A. Amyloid heart disease mimicking hypertrophic cardiomyopathy. J Intern Med 2005;258:225–30.
- Koyama J, Ray-Sequin PA, Davidoff R, Falk RH. Usefulness of pulsed tissue Doppler imaging for evaluating systolic and diastolic left ventricular dysfunction in patients with AL (primary) amyloidosis. Am J Cardiol 2002;89:1067–71.
- Maceira AM, Joshi J, Prasad SK. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 2005;111:195–202.
- Hawkins PN, Lavender JP, Pepys MB. Evaluation of systemic amyloidosis by scintigraphy with labelled 123I-labelled serum amyloid P component. N Engl J Med 1990;323:508–13.
- Liao R, Jain M, Teller P et al. Infusions of light chains from patients with cardiac amyloidosis causes diastolic dysfunction in mouse hearts. Circulation 2001;104:1594–7.
- Brenner DA, Jain M, Pimentel DR et al. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res 2004;94:1008–10.
- Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Seminars in Hematology 1995;32:45–59.
- Kyle RA, Gertz MA, Greipp PR et al. Long term survival (10 years or more) in 30 subjects with primary amyloidosis. Blood 1999;93:1062–6.
- Dubrey S, Cha K, Chamarsee B, Anderson J, Skinner M, Falk RH. Primary (AL) cardiac amyloidosis: symptoms, signs and non-invasive investigations in 232 patients. Q J Med 1998;91:141–57.
- Lachmann HJ, Booth DR, Booth SE et al. Misdiagnosis of hereditary amyloidosis as AL (primary) amyloidosis. N Engl J Med 2002;346:1786–91.
- Guidelines on the diagnosis and management of AL amyloidoisis. Br J Haematol 2004:125:681–700.
- Sanchorawala V, Seldin DC, Magnani B, Skinner M, Wright DG. Serum free light chain responses after high-dose intravenous melphalan and autologous stem cell transplantation for AL (primary) amyloidosis. Bone Marrow Transplant 2005;36:597–600.
- Lachmann HJ, Gallimore R, Gillmore JD et al. Outcome in systemic AL amyloidosis in relation to changes in concentration of circulating free immunoglobulin light chains following chemotherapy. Br J Haematol 2003;122:78–84.
- Comenzo RL, Vosburgh E, Falk RH et al. Dose-intensive melphalan with blood stem-cell support for the treatment of AL (amyloid light chain) amyloidosis: survival and responses in 25 patients. Blood 1998;91:3662–70.
- Skinner M, Sanchorawala V, Seldin DC et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8 year study. Ann Int Med 2004;140:85–93.
- Mollee PN, Wechalekar AD, Pereira DL et al. Autologous stem cell transplantation in primary systemic amyloidosis: the impact of selection criteria on outcome. Bone Marrow Transplant2004;33:271–7.
- Seldin DC, Choufani EB, Dember LM et al. Tolerability and efficacy of thalidomide for the treatment of subjects with light chain-associated (AL) amyloidosis. Clinical Lymphoma 2003;3:241–6.
- Dispenzieri A, Lacy MQ, Rajkumar SV et al. Poor tolerance to high doses of thalidomide in subjects with primary systemic amyloidosis. Amyloid 2003;10:257–61.
- Palladini G, Perfetti V, Perlini S et al. The combination of thalidomide and intermediate-dose dexamethasone is an effective but toxic treatment for patients with primary amyloidosis (AL). Blood2005;105:2949–51.
- Hussein MA, Juturi JV, Rybicki L, Lutton S, Murphy BR, Karam MA. Etanercept therapy in patients with advanced primary amyloidosis. Med Oncol 2003;20:283–90.
- Gianni L, Bellotti V, Gianni AM, Merlini G. New drug therapy of amyloidosis: resorption of AL-type deposits with 4’-iodo-4’deoxydoxorubicin. Blood 1995;86:855–61.
- Merlini G, Anesi E, Garini P et al. Treatment of AL amyloidosis with 4’-iodo-4’deoxydoxorubicin: an update. Blood 1999;93:1112–13.
- Gertz MA, Lacy MQ, Dispenzieri A et al. A multicenter phase II trial of 4’-iodo-4’deoxydoxorubicin (IDOX) in primary amyloidosis (AL). Amyloid 2002;9:24–30.
- Dubrey SW, Burke MM, Hawkins PN, Banner NR. Cardiac transplantation for amyloid heart disease – the United Kingdom experience. J Heart Lung Transplantation 2004;23:1142–53.
- Hosenpud JD, DeMarco T, Frazier OH et al. Progression of systemic disease and reduced long term survival in patients with cardiac amyloidosis undergoing heart transplantation. Circulation1991;84(5 Suppl):III338–III343.
- Dubrey S, Cha K, Skinner M, LaValley M, Falk RH. Familial and primary (AL) cardiac amyloidosis: echocardiographically similar diseases with distinctly different clinical outcomes. Heart1997;78:74–82.
- Holmgren G, Ericzon BG, Groth CG et al. Clinical improvement and amyloid regression after liver transplantation in hereditary transthyretin amyloidosis. Lancet 1993;341:1113–16.
- Zeldenrust S, Gertz M, Uemichi T et al. Orthotopic liver transplantation for hereditary fibrinogen amyloidosis. Transplantation 2003;75:560–1.
- Dubrey SW, Davidoff R, Skinner M, Bergethon P, Lewis D, Falk R. Progression of ventricular wall thickening after liver transplantation for familial amyloidosis. Transplant 1997;64:74–80.
- Yazaki M, Tokuda T, Nakamura A et al. Cardiac amyloid in patients with familial amyloid polyneuropathy consists of abundant wild-type transthyretin. Biochem Biophys Res Commun2000;274:702–06.
- Westermark P, Sletten K, Johansson B, Cornwell GG III. Fibril in senile systemic amyloidosis is derived from normal transthyretin. Proc Natl Acad Sci USA 1990;87:2843–5.
- Kyle RA, Spittell PC, Gertz MA et al. The premortem recognition of systemic senile amyloidosis with cardiac involvement. Am J Med 1996;101:395–400.
- Ng B, Connors LH, Davidoff R, Skinner M, Falk RH. Senile systemic amyloidosis presenting with heart failure: a comparison with light chain-associated amyloidosis. Arch Intern Med2005;165:1425–9.
- Dubrey SW, Cha K, Simms RW, Skinner M, Falk RH. Electrocardiography and Doppler echocardiography in secondary (AA) amyloidosis. Am J Cardiol 1996;77:313–15.
- Kaye GC, Butler MG, d’Ardenne AJ, Edmondson SJ, Camm AJ, Slavin G. Isolated atrial amyloid contains atrial natriuretic peptide: a report of six cases. Br Heart J 1986;56:317–20.
- Kawamura S, Takahashi M, Ishihara T, Uchino F. Incidence and distribution of isolated atrial amyloid: histologic and immunohistochemical studies of 100 aging hearts. Pathol Int 1995;45:335–42.
