Alcohol septal ablation (ASA) is an alternative therapeutic method to the gold-standard surgical myectomy in the treatment of symptomatic left ventricular outflow tract (LVOT) obstruction in patients with hypertrophic cardiomyopathy (HCM). ASA is performed by injecting alcohol into the target septal branch of the left anterior descending coronary artery. In this article, we review the rationale and indications for ASA, provide a practical description of the technique and give an overview of the published data placing it in context with the surgical approach. We also report our experience of the technique in a typical sample of patients referred to a tertiary centre providing demographic, echocardiographic and clinical outcomes data during an average follow-up period of three years. Our data confirm that ASA is an effective non-surgical technique for treatment of symptoms related to LVOT obstruction in HCM. Medium-term follow-up demonstrates persistent reduction in LVOT obstruction and improvement in New York Heart Association (NYHA) functional class. Long-term studies of larger populations are necessary to determine the wider prognostic significance of the procedure.
Introduction
Hypertrophic cardiomyopathy (HCM) is an inherited cardiac muscle disorder, transmitted predominantly in an autosomal dominant fashion with variable penetrance and an estimated prevalence of one in 500 in the western population.1 Myocardial hypertrophy most commonly affects the interventricular septum and may be associated with dynamic obstruction of the left ventricular outflow tract (LVOT),2 caused by systolic anterior motion (SAM) of the mitral valve leaflets. LVOT obstruction may occur at rest or only become apparent during physiological stress. Symptoms related to LVOT obstruction include dyspnoea, exertional chest pain, pre-syncope or syncope. Conventional medical treatment for symptoms related to outflow obstruction includes beta blockers, calcium channel antagonists, and occasionally disopyramide. Dual-chamber pacemaker implantation has also been shown to have some symptomatic benefit, although blinded crossover studies suggested this benefit was limited to elderly patients.3 Surgical myectomy has been established as the primary treatment modality to relieve outflow tract obstruction associated with severe drug-refractory heart failure symptoms and marked functional disability (New York Heart Association [NYHA] class III/IV).
Alcohol septal ablation (ASA), first reported in 1995 by Sigwart,4 is an alternative therapeutic method to surgical myectomy for the treatment of LVOT obstruction. ASA is performed by injection of absolute alcohol into the septal branch of the left anterior descending (LAD) coronary artery producing a ‘therapeutic infarction’ within the basal ventricular septum. The localised myocardial necrosis leads to progressive scarring and a subsequent thinning of the basal septal region, expanding the LVOT. This reduces or eliminates SAM, ultimately leading to a reduction in sub-aortic obstruction4-6 and mitral regurgitation, mimicking the haemodynamic consequences of surgical septal myectomy.7,8 Similar outcomes in terms of improvement in symptoms and relief of LVOT obstruction have been reported for both ASA and surgical myectomy, although the incidence of permanent pacemaker implantation is generally greater after ASA.9,10
Indications for alcohol septal ablation
The American College of Cardiology and European Society of Cardiology (ACC/ESC) published joint guidance on the management of hypertrophic cardiomyopathy in 2003.11 The indications for ASA in these guidelines are widely accepted as follows: (a) severe heart failure symptoms (NYHA class III or IV) refractory to appropriate medical therapy, associated with a (b) sub-aortic gradient of 50 mmHg (peak) or greater, measured with Doppler echocardiography either at rest and/or during physiological exercise. The outflow gradients should either be secondary to SAM or proximal mitral valve-septal contact.
Technique
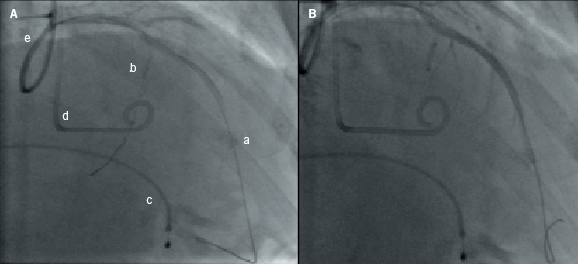
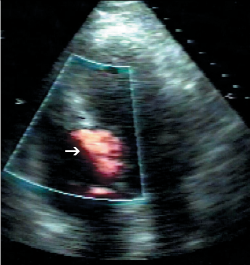
This section provides a descriptive account of the procedure as performed in our centre. All cases were performed via the femoral arterial approach such that a 6-French sheath was placed in the right femoral artery and 5-French sheath in the left side. In those without a permanent pacemaker (PPM), a 5-French venous sheath was also inserted to allow back-up temporary pacing of the right ventricle. Diagnostic coronary angiography was then performed to exclude significant coronary artery disease and to assess the septal branch as a potential target for alcohol ablation. A pigtail catheter was then advanced into the mid-cavity of the left ventricle for the measurement of intra-cavity pressure. A 6-French guide catheter was used to cannulate the left coronary artery and to obtain simultaneous aortic pressures to allow continuous assessment of the LVOT gradient. A long guidewire was then passed into the selected proximal septal branch and a separate guide wire placed into the LAD (figure 1A). An over-the-wire balloon was then inflated in the septal branch just beyond its origin to prevent the spillage of alcohol into the LAD. The guidewire in the septal branch was removed and contrast medium injected through the balloon lumen to delineate the area supplied by the septal branch and to ensure no retrograde flow into the LAD (figure 1B). In selected cases, myocardial contrast echocardiography (MCE) was performed, in order to echocardiographically delineate the myocardial territory supplied by the chosen septal branch (figure 2). This was followed by slow instillation of absolute alcohol (volume administered depending on the septal thickness and branch size) over two to five minutes. The LVOT gradient and electrocardiogram (ECG) were monitored continuously. The temporary wire was left in-situ for 24–48 hours post-procedure and patients were closely monitored for at least 48 hours. Serial measurements of cardiac enzymes (creatine kinase; CK) were made at 12, 24 and 48 hours post-procedure.
Manchester Heart Centre experience
The technique of ASA has been performed in our centre since January 2004. Patients were considered eligible for ASA in accordance with ACC/ESC guidelines. All patients underwent baseline clinical assessment, as well as transthoracic echocardiography for the assessment of left ventricular (LV) dimensions and function, left atrial (LA) dimension, LVOT gradient, and presence of SAM. Standard echocardiographic images were acquired and stored digitally as looped cardiac cycles for later offline analysis. Exercise echocardiography was performed where clinically indicated using treadmill exercise according to a standard Bruce protocol with immediate post-exercise echocardiographic assessment of the LVOT gradient. Follow-up clinical assessment and echocardiography were performed at three months, and six to nine months post-procedure. Cardiac dimensions were measured according to American Society of Echocardiography (ASE) guidelines. Clinical outcomes were analysed by retrospective case note review.
Procedural results
The demographic data of a total of 18 patients considered eligible for ASA are shown in table 1. None of the patients had a family history of sudden cardiac death. Fifteen patients underwent ASA, and in the remaining three cases the procedure was not performed because of unsuitable septal branch anatomy. A mean of 4.75 ml (range 1.5–9.0 ml) of absolute alcohol were injected into the septal perforator artery. Two patients developed transient right bundle branch block (RBBB) and five had transient broadening of the QRS complex, and in all these patients the ECG normalised within 72 hours. Three patients developed complete heart block in the peri-operative period requiring PPM implantation. One patient had a localised dissection of the septal branch of the LAD; the procedure was terminated and a repeat procedure was successful three months later. One patient had a small pericardial effusion, which was managed conservatively. One patient had a right coronary artery stenosis that required percutaneous coronary intervention (PCI); however, breathlessness persisted and the patient then underwent ASA. There was no peri-procedural death. The average duration of in-patient stay was 4.5 ± 2.9 days (range 2–15). Three patients needed PPM implantation due to persistent complete heart block more than five days post-procedure. The average CK level was 1,180 U/L (range 192–2,863 U/L) 12 hours after ablation.
Echocardiographic follow-up
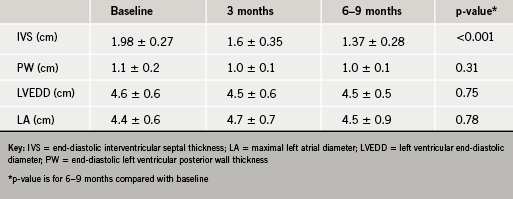
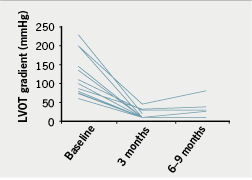
There was a significant reduction in ventricular septal thickness within three months of ablation, which continued at six to nine months (table 2). There was also significant improvement in the resting LVOT gradient (figure 3) with a mean at presentation of 98 ± 54 mmHg, improving to 19 ± 17 mmHg within three months of ablation (p<0.001). In 10 out of 15 patients, no resting LVOT gradient was detectable at follow-up. The improvement in LVOT gradient was maintained at six to nine months follow-up (mean gradient 22 ± 21 mmHg, p<0.001 compared with baseline). There was also an observed reduction in SAM and associated mitral regurgitation in 60% of cases. There was no significant change in LV end-diastolic dimension (4.6 ± 0.6 cm vs. 4.5 ± 0.5 cm, p=0.75) or left atrial dimension (4.4 ± 0.6 cm vs. 4.5 ± 0.9 cm, p=0.78) over the course of the study.
Clinical follow-up
Mean duration of follow-up was 36 ± 18 months (range 6–60 months). There was no early (within 30 days) mortality; one patient died three months after the procedure due to a septicaemia secondary to chest infection. During short-term follow-up (three months), 13 out of 15 patients reported significant symptomatic improvement with an associated reduction in the NYHA class as shown in figure 4. Three patients with persistent symptoms at three months follow-up had LVOT gradients of 1, 5 and 27 mmHg, compared with pre-procedure gradients of 100, 50 and 75 mmHg, respectively.
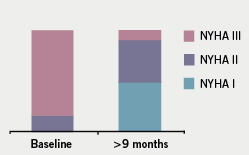
The clinical improvement was sustained in the medium term with 10 (67%) patients remaining in NYHA class I–II at nine months. None of the patients had a repeat procedure or required re-admission for cardiac events. Two patients had evidence of paroxysmal atrial fibrillation on ambulatory monitoring post-ablation and eight patients had ambulatory monitoring that did not reveal any arrhythmias. Sixteen patients continued treatment with low-dose beta blocker and one with a calcium antagonist. The three patients who did not undergo ASA were managed conservatively; no patients were referred for surgical myectomy.
Discussion
Patients with HCM associated with symptomatic LVOT obstruction have an impaired quality of life and adverse prognosis.12 Although the presence of LVOT obstruction is an independent predictor of progression to severe heart failure and of death, to date no evidence exists of prognostic benefit associated with the relief of obstruction.
The main finding of our study is that ASA is a well-tolerated and efficacious procedure with few in-hospital complications, even in a relatively elderly group of patients. None of the patients suffered major peri-procedural complications such as in-hospital death, ventricular arrhythmias, cardiac tamponade or distant infarction. Three patients needed permanent pacing, which is consistent with the rates in the published literature.13,14
We also confirm that reduction in septal wall thickness, and consequently LVOT obstruction, continues over the medium term, related to progressive maturation of the ASA-induced scar within the ventricular septum and the associated remodelling of LVOT geometry. The maximum outflow gradient reductions following septal ablation occurred between three and 12 months. Consequently, for those in whom symptoms fail to improve, consideration of a further procedure should be deferred for at least six months. This re-assessment should also include a search for other potential causes of persistent breathlessness, such as diastolic dysfunction, arrhythmias or lung disease. Although none of our patients had a repeat procedure, re-intervention rates in the range of 5% have been reported.15
Concern has previously been raised regarding the potential for an increased rate of scar-related arrhythmia post-ASA13,14 and adverse global left ventricular remodelling. None of our patients had clinical or Holter evidence of ventricular arrhythmias during follow-up. Moreover, there were no significant changes in left ventricular dimensions, suggesting that post-infarct remodelling remains confined to the LVOT. Obstructive HCM may also be associated with progressive left atrial enlargement due to diastolic dysfunction. Left atrial enlargement >46 mm is a marker of disease severity16 and an independent predictor of death in patients undergoing surgical myectomy.17 In our study, the LA dimension did not change significantly and, although the follow-up period was relatively short, the absence of progressive LA dilatation might suggest an improvement in left ventricular diastolic function following relief of obstruction.
According to the ACC/ESC guidelines, septal myectomy should remain the gold-standard treatment, and the use of ASA should be confined to older adults in an attempt to limit the adverse effect of any small increase in the risk of serious arrhythmia. The average age of patients in our study is consistent with this recommendation.
Study limitations
This study consists of a relatively small sample size, reflecting the fact that these data are derived from real-world practice in a highly selected group of patients. Although no major adverse cardiac events were detected during follow-up, assessment of arrhythmias by ambulatory monitoring was not performed on a systematic basis. Therefore, asymptomatic or transient arrhythmias may have been missed, although the exact relevance of this remains unclear.
Conclusions
Our results demonstrate that ASA performed according to current guidelines in a real-world tertiary referral population is a beneficial interventional treatment modality for patients with obstructive HCM refractory to medical treatment. The effect of ASA on long-term prognosis remains to be determined in larger studies with longer follow-up periods.
Conflict of interest
None declared.
Editors’ note
Please see also the editorial by Sekhri et al. on pages 201–02 of this issue.
Key messages
- Alcohol septal ablation (ASA) is an alternative therapeutic method in the treatment of symptomatic left ventricular outflow tract (LVOT) obstruction in patients with hypertrophic cardiomyopathy (HCM)
- ASA is performed by injecting alcohol into the target septal branch of the left anterior descending coronary artery
- Our experience of the technique in a typical sample of patients referred to a tertiary centre confirms that ASA is an effective non-surgical technique for treatment of symptoms related to LVOT obstruction in HCM
- Long-term studies of larger populations are necessary to determine the wider prognostic significance of the procedure
References
1. McKenna WJ, Behr ER. Hypertrophic cardiomyopathy: management, risk stratification, and prevention of sudden death. Heart 2002;87:169–76. (doi: 10.1136/heart.87.2.169)
2. Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet 2004;363:1881–91. (doi: 10.1016/S0140-6736(04)16358-7)
3. Maron BJ, Nishimura RA, McKenna WJ et al. Assessment of permanent dual-chamber pacing as a treatment for drug-refractory symptomatic patients with obstructive hypertrophic cardiomyopathy: a randomized, double-blind, crossover study (M-PATHY). Circulation 1999;99:2927–33.
4. Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet 1995;346:211–14. (doi: 10.1016/S0140-6736(95)91267-3)
5. Braunwald E. Induced septal infarction: a new strategy for hypertrophic obstructive cardiomyopathy. Circulation 1997;95:1981–2.
6. Seggewiss H, Gleichmann U, Faber L et al. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: acute results and 3-month follow-up in 25 patients. J Am Coll Cardiol 1998;31:252–8. (doi: 10.1016/S0735-1097(97)00508-1)
7. Faber L, Meissner A, Ziemsenn P, Seggewiss H. Percutaneous transluminal septal myocardial ablation for hypertrophic obstructive cardiomyopathy: long term follow up of the first series of 25 patients. Heart 2000;83:326–31. (doi: 10.1136/heart.83.3.326)
8. Gietzen FH, Leuner CJ, Raute-Kreinsen U et al. Acute and long-term results after transcoronary ablation of septal hypertrophy (TASH). Catheter interventional treatment for hypertrophic obstructive cardiomyopathy. Eur Heart J 1999;20:1342–54. (doi: 10.1053/euhj.1999.1520)
9. Alam M, Dokainish H, Lakkis NM. Hypertrophic obstructive cardiomyopathy-alcohol septal ablation vs. myectomy: a meta-analysis. Eur Heart J 2009;30:1080–7. (doi: 10.1093/eurheartj/ehp016)
10. Qin JX, Shiota T, Lever HM et al. Outcome of patients with hypertrophic obstructive cardiomyopathy after percutaneous transluminal septal myocardial ablation and septal myectomy surgery. J Am Coll Cardiol 2001;38:1994–2000.
11. Maron BJ, McKenna WJ, Danielson GK et al. American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on Hypertrophic Cardiomyopathy a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol 2003;42:1687–713. (doi: 10.1016/S0735-1097(03)00941-0)
12. Maron MS, Olivotto I, Betocchi S et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med 2003;348:295–303. (doi: 10.1056/NEJMoa021332)
13. Alam M, Dokainish H, Lakkis NM. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a systematic review of published studies. J Interv Cardio 2006;19:319–27. (doi: 10.1111/j.1540-8183.2006.00153.x)
14. Sorajja P, Nishimura RA, Gersh BJ et al. Outcome of mildly symptomatic or asymptomatic obstructive hypertrophic cardiomyopathy: a long-term follow-up study. J Am Coll Cardiol 2009;54:234–41. (doi: 10.1016/j.jacc.2009.01.079)
15. Kuhn H, Seggewiss H, Gietzen FH et al. Catheter-based therapy for hypertrophic obstructive cardiomyopathy. First in-hospital outcome analysis of the German TASH Registry. Kardiol 2004;93:23–31. (doi: 10.1007/s00392-004-1028-6)
16. Nagueh SF, Lakkis NM, Middleton KJ et al. Changes in left ventricular filling and left atrial function six months after nonsurgical septal reduction therapy for hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol 1999;34:1123–8. (doi: 10.1016/S0735-1097(99)00341-1)
17. Woo A, Williams WG, Choi R et al. Clinical and echocardiographic determinants of long-term survival after surgical myectomy in obstructive hypertrophic cardiomyopathy. Circulation 2005;111:2033–41. (doi: 10.1161/01.CIR.0000162460.36735.71)
