Achieving a lower heart rate is important in treating angina. Established approaches include the use of beta blockers and certain calcium channel blockers. However, the use of these drugs may be limited by side effects or contraindications. Ivabradine (Procoralan®) is a novel agent that lowers heart rate through selective I(f) channel inhibition, acting specifically on the sinus node. We present a consecutive series of 30 patients initiated on ivabradine, within a district general hospital (DGH) setting. The aim of this study was to identify the heart rate-lowering and symptom-control properties of ivabradine, while monitoring adverse effects. Heart rate was measured on a baseline electrocardiogram (ECG) prior to starting ivabradine, and then within a 12-month follow-up period. The results identified a mean (standard deviation) 10 (14) beats per minute (bpm) decrease achieved on ivabradine (p<0.001), with greatest reduction in heart rate in those with a resting heart rate over 80 bpm prior to starting treatment (p<0.05), and in patients on a 5 mg twice-daily dosing regimen at follow-up (p<0.05). In parallel, the majority of patients reported favourable symptom benefit (21/30), and low rate of adverse events with discontinuation rate of only 2/30 felt directly related to the drug itself. We believe this to be the first report of using this novel drug in a ‘real-world’ DGH setting. The findings add confidence in using this anti-anginal agent in appropriate patients, and furthermore support conducting studies involving multiple centres, to further define and assess ivabradine in the clinical setting of angina.
Introduction
An elevated heart rate may be a primary determinant of myocardial ischaemia by altering the balance of oxygen demand and coronary perfusion. Given that there is considerable evidence showing survival is inversely related to heart rate, lowering heart rate would be expected to be an important tool in the management of angina.1-3 Theoretically it may also be beneficial in the prevention of myocardial infarction as the haemodynamic stresses placed upon the myocardium by a high heart rate are associated with coronary plaque rupture.4
Approaches to lowering heart rate include the use of beta blockers and certain calcium channel blockers; however, their use may be limited by side effects such as fatigue, depression, ankle swelling, sexual dysfunction, and beta blockers are contraindicated in those with airways disease.
Heart rate is regulated through the I(f) channel, which determines spontaneous electrical pacemaker activity within the sinoatrial node. Ivabradine lowers heart rate by selective I(f) channel inhibition, with evidence to suggest superior anti-anginal and anti-ischaemic activity to placebo and is equivalent to atenolol and amlodipine in symptom relief.5,6
We present a consecutive series of 30 patients, initiated on ivabradine, with follow-up on the efficacy of heart rate reduction, anginal symptom control and reporting of adverse effects. We believe this to be the first report of using this novel drug in a ‘real-world’ setting at a busy district general hospital (DGH) over a 12-month period.
Methods
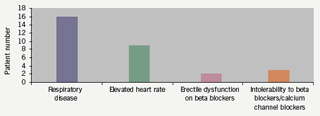
Thirty patients, from either out-patients or those stable as medical in-patients, were included over a 12-month period beginning March 2006. Ages ranged from 52 to 87 years (mean 71.3 years), with 20 males and 10 females. The indication for commencing ivabradine therapy was an elevated heart rate despite receiving anti-anginals (target resting heart rate of 60 beats per minute [bpm]) and/or intolerant/not suitable for beta blockers and calcium channel blockers (figure 1). A 12-lead resting electrocardiogram (ECG) was available prior to starting ivabradine. A follow-up ECG was subsequently performed after mean (standard deviation [SD]) duration 12.6 (8) weeks post ivabradine therapy, at which time the patient completed a questionnaire on symptoms and adverse effects. For further clarification a number of telephone enquiries were also conducted. Patients were commenced on either 2.5 mg or 5 mg twice- daily regimens, with a view to uptitration in primary care and clinic follow-up.
Results
Patients
The majority of patients were from cardiology out-patients (n=20), but also included a small number of stable in-patients (n=10). Twenty men and 10 women took part in the study. The majority of the patients were of Caucasian origin (n=18). The mean age for men was 72 years and for women it was 71.3 years. Mean duration of follow-up was 12.6 weeks. The mean total number of medications among the patients was 8.5.
Effect on heart rate (figure 2)
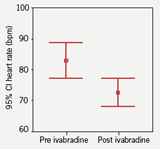
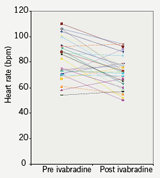
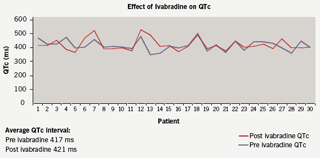
Heart rate was compared pre and post ivabradine therapy with 12-lead resting ECGs. Prior to starting therapy the mean heart rate was 83 bpm. On therapy the heart rate was reduced to 73 bpm which represented a mean (SD) 10 (14) bpm decrease in rate (p<0.001; figure 3), with no change in corrected QT interval (figure 4). At follow-up, 10 patients remained on the 2.5 mg dosing regimen. A heart rate reduction of 6 bpm was seen in this group comparing pre and post ivabradine heart rate (p=0.354). In comparison, in the 20 patients who were on 5 mg twice-daily at follow-up, a 12 bpm mean decrease in heart rate was observed (p<0.05), which encourages the role for uptitration. It was noted that 14 patients had a pre ivabradine resting heart rate of under 80 bpm (mean [SD] 69 [6] bpm).
The remaining 16 patients all had pre ivabradine resting heart rates of over 80 bpm (mean [SD] 95 [8] bpm). Interestingly, the greatest heart rate reduction was observed in patients who had higher resting heart rates to begin with (over 80 bpm). A significant 19 bpm decrease in mean pre and post ivabradine heart rates was seen in this subgroup (p<0.05).
Symptom control/adverse effects
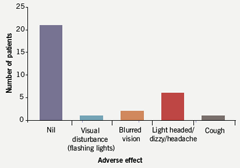
Patients completed a questionnaire to assess anginal symptoms and adverse effects while on ivabradine. Twenty-one patients reported their angina as being stable or better (70%) (figure 5). Nine reported it as worse. Side effect profile was favourable with the majority of patients reporting no adverse effects. Of note, three patients described visual disturbances of which one reported flashing lights, a known side effect of ivabradine. Subsequently this patient ceased taking ivabradine upon request by her general practitioner (GP). Two patients reported blurred visual symptoms, which were also present prior to starting ivabradine. One of these two patients is known to have diabetic eye disease. Overall, the side effect profile did not adversely affect tolerability or daily patient activities, and the discontinuation rate related to the drug itself was 2/30 (figure 6).

Discussion
We report a first experience of using ivabradine in a single centre DGH clinical setting. The 10 bpm decrease in heart rate observed in this series correlates with previous reports. A reduction of 4.5 and 9.5 bpm, at 2.5 mg and 5.0 mg twice-daily dosing, respectively, has been shown when ivabradine is used as monotherapy.7 When used in combination with calcium channel blockers and nitrates, a 12-month, double-blind study, showed reduction of heart rate by 10 and 12 bpm, respectively, for 5.0 mg and 7.5 mg doses.8
In this study the greatest heart rate reduction was seen in those patients with higher resting heart rates prior to starting ivabradine. A mean reduction of 19 bpm was observed in those with resting heart rates of over 80 bpm prior to ivabradine therapy, which may well represent a plateauing of rate characteristic of this drug.
At follow-up the patients completed a questionnaire on ivabradine. The majority of the patients reported their angina as being stable or better. The adverse effects profile was low without consequences upon quality of life. Two patients in total discontinued ivabradine at follow-up.
The limitations of this study should be noted. The study was not double-blinded and was conducted in a relatively small population (n=30) – highlighting the only recent introduction of ivabradine into Hospital Trust pharmacy. The possible influences of polypharmacy (mean number of total medications 8.5), old age, and the recommended cautious starting dose of ivabradine when this study was started being 2.5 mg twice-daily in those above 75 years, should be borne in mind. It was noted that a significant reduction in heart rate was seen in patients who were on 5 mg twice-daily at follow-up (n=20), as opposed to 2.5 mg twice-daily (n=10) highlighting the importance of uptitration.
Questionnaires were used to elicit symptom control and adverse effects – which may be potentially flawed as they are simply qualitative and potentially inaccurate if polypharmacy is common. A more objective method would be to formally assess their symptoms in the form of an ECG treadmill test. This dynamic and more robust objective interpretation of symptoms has been described recently by Ruzyllo et al.6 Their large study comparing ivabradine to amlodipine used bicycle exercise tolerance at baseline and at monthly intervals looking at duration of exercise, heart rate, ECG changes, and nitrate usage. It showed ivabradine had comparable efficacy to amlodipine in improving exercise tolerance and superior effects on the reduction of myocardial oxygen demand.6
In conclusion, we looked at a series of patients with angina under the care of a cardiology service at a busy East London DGH. The reduction in heart rate and the favourable symptoms/side effects profile we observed adds confidence in the therapeutic role of ivabradine. This small study correlates well with previous data highlighting the heart rate-lowering properties of ivabradine – providing confidence in its role in anginal management – and will pave the way for more robust prospective studies, ideally involving several cardiac units. These data should be supplemented by data expected in autumn 2008 from the morBidity-mortality EvAlUaTion of the I(f) inhibitor ivabradine in patients with coronary disease and left ventricULar dysfunction (BEAUTIFUL) study,9 which will be the first major clinical outcome trial of ivabradine.
Conflict of interest
SG has received travel expenses and honoraria related to lectures on ivabradine, through Servier (UK and Worldwide) Ltd; and also sat on an Advisory Panel for Angina, organised by Servier UK.
Key messages
- Mean heart rate reduction of 10 beats per minute was achieved on ivabradine therapy (p<0.001). The majority of patients reported their angina as stable or improved (21/30), with minimal adverse effects (discontinuation rate 2/30)
- Greatest heart rate reduction was seen in those patients who had high resting heart rates (over 80 beats per minute) prior to starting ivabradine
- Significant heart rate reduction was observed in those patients who were on a 5 mg twice-daily regimen at follow-up versus 2.5 mg twice-daily
- Future studies should look at larger population groups, with dynamic testing upon heart rate
References
- Sulfi S, Timmis A. Ivabradine – the first selective sinus node If channel inhibitor in the treatment of stable angina. Int J Clin Pract 2006;60:222–8.
- Habib G. Is heart rate a risk factor in the general population? Dialogues Cardiovascular Medicine 2001;6: 25–31.
- Singh BN. Impact of heart rate on cardiovascular disorders: focus on chronic stable angina. In: Fox K, ed. Selective and specific If channel Inhibition in cardiology. London: Science Press Ltd, 2004:25–36.
- Heidland UE, Strauer BE. Left ventricular mass and elevated heart rate are associated with coronary plaque disruption. Circulation 2001;104:1477–82.
- Tardif JC, Ford I, Tendera M, Bourassa MG, Fox K. Efficacy of ivabradine, a new selective If inhibitor compared with atenolol in patients with chronic stable angina. Eur Heart J 2005;26:2529–36.
- Ruzyllo W, Tendera M, Ford I, Fox KM. Antianginal efficacy and safety of ivabradine compared with amlodipine in patients with stable effort angina pectoris: a 3-month randomised, double-blind, multicentre, noninferiority trial. Drugs 2007;67:393–405.
- Borer JS, Fox K, Jaillon P, Lerebours G. Anti-anginal and anti-ischemic fects of ivabradine, an If inhibitor, in stable angina: a randomized, double-blinded, multicentered, placebo-controlled trial. Circulation 2003;107:817–23.
- Lopez-Bescos L, Filipova S, Martos R. Long-term safety and antianginal efficacy of the If current inhibitor ivabradine in patients with chronic stable angina. A one-year randomized, double-blind, multicentre trial. Eur Heart J 2004;25:878.
- Fox K, Ferrari R, Tendera M, Steg PG, Ford I; BEAUTIFUL Steering Committee. Rationale and design of a randomized, double-blind, placebo-controlled trial of ivabradine in patients with stable coronary artery disease and left ventricular systolic dysfunction: the morBidity-mortality EvAlUaTion of the I(f) inhibitor ivabradine in patients with coronary disease and left ventricULar dysfunction (BEAUTIFUL) study. Am Heart J 2006;152:860–6.
