The role of cardiac computed tomography (CT) in clinical practice is constantly evolving. Early machines were only capable of measuring coronary calcification. Advances in temporal and spatial resolution, especially the introduction of 64-detector rows, now mean that high-quality non-invasive angiograms are possible in most patients. This review will outline the capabilities and limitations of coronary artery imaging with CT, and also highlight areas that differentiate CT from X-ray angiography, including direct plaque visualisation and potential vulnerable plaque identification.
Development of cardiac computed tomography
The concept of ‘computerised transverse axial scanning’ was first demonstrated by Godfrey Hounsfield nearly 30 years ago.1 Initial computed tomography (CT) scanners required up to 300 seconds for the acquisition of a single image. With such poor temporal resolution they were only suitable for imaging static structures such as the brain.2 The coronary arteries move throughout the cardiac cycle, although their velocity decreases in diastole.3 This underlies the concept of ‘gating’ the scan with the electrocardiogram (ECG), so that data are acquired preferentially during diastole.4 The advent of multi-detector CT (MDCT) has allowed simultaneous acquisition of multiple slices of imaging data. Current CT scanners can deliver a temporal resolution of 75 ms (due to very rapid gantry rotation5) at a spatial resolution of under 400 µm.5
Coronary artery calcium
The advent of electron beam CT (EBCT) in the 1980s gave sufficient temporal resolution to image the coronary arteries.6 Although it could not quantify luminal stenosis, it did allow reliable identification of coronary artery calcification (CAC).7 CT provides good visualisation of vascular calcification because of the marked X-ray attenuation properties of calcium. CAC occurs almost exclusively as a consequence of atherosclerosis and so its presence is a sensitive marker of the atherosclerotic disease process.8 Calcification does not necessarily concentrate at the site of maximal stenosis, so cannot be used to diagnose obstructive coronary disease.9 Instead, the total amount of calcification provides a surrogate measure of the total plaque burden. This burden can be quantified as the ‘Agatston score’, after its pioneer Arthur Agatston.
The CAC score is measured without contrast and is a low radiation scan (1–2 mSv). Multiple large prospective outcome studies of CAC scoring have confirmed the prognostic importance of coronary artery calcium. In a registry of over 25,000 patients, a calcium score of 0 conferred a very low event rate, with 12-year survival of 99.4%.10 A meta-analysis of six studies revealed that an increasing CAC score meant incremental increases in the relative risk (RR) of myocardial infarction (MI) or cardiac death at 3–5 years compared to a zero score: (CAC 1–112 = RR 1.9, 100–400 = 4.3, 400–999 = 7.2 and >1000 = 10.8).11 EBCT has now been superseded by MDCT, but the prognostic message remains the same (figure 1). It should be noted, however, that CAC scoring is not suitable for indiscriminate screening of asymptomatic patients. The likelihood of finding coronary atherosclerosis in low-risk (by Framingham or other scores) patients is too low to warrant imaging, and patients at high risk require risk factor modification irrespective of the result. CAC scoring is most useful in asymptomatic patients at intermediate risk of disease, where a high score will re-classify patients into the high-risk category leading to intensive risk factor modification.12 The National Institute for Health and Clinical Excellence (NICE) have constructed guidelines on the role of CAC and CT coronary angiography (CTCA) in the investigation of symptomatic patients. They suggest patients with suspected cardiac chest pain without confirmed coronary artery disease (CAD) in whom the estimated likelihood of CAD is 10–29% (low-to-intermediate risk) should be offered CAC scoring. If the CAC is 0, other causes of chest pain should be sought. If the CAC is 1–400, then 64-slice CTCA should be offered. If the CAC score is >400, then invasive angiography should be offered if clinically appropriate.13 There are caveats; calcified plaque only represents approximately 20% of the total coronary atherosclerotic burden.14 One series has shown that 4% of symptomatic patients with a reassuring CAC score of 0 had significant stenosis defined at invasive angiography. This can occur when plaques are composed entirely of non-calcified elements.15 The positive correlation seen between CAC scores and cardiac event rates is probably secondary to the increased amounts of non-calcified plaque that accompany the calcified plaque, rather than the direct pathological involvement of the calcified plaques themselves.
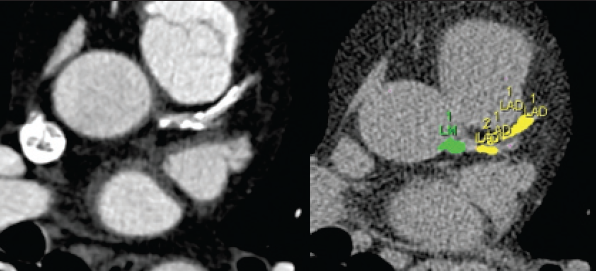
CTCA
In a recently published study examining nearly 400,000 patients who underwent invasive coronary angiography for stable chest pain, 38% were found to have no obstructive coronary disease.16 As invasive angiography has a serious complication rate of one in 1,000, it is desirable for a non-invasive test to replace some of these ‘negative’ procedures. CTCA is widely available and provides the possibility of fulfilling this requirement (figure 2).17
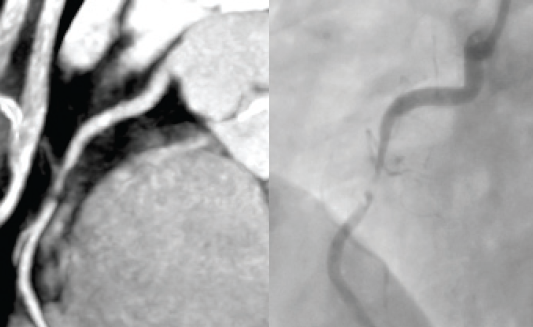
CTCA is best performed in those in sinus rhythm. Beta blockers and sublingual nitrates are routinely administered. High or irregular heart rates and heavy calcification all reduce the diagnostic accuracy of the technique.18
Several comparator studies of CTCA and invasive angiography have recently reported. The Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography (ACCURACY) study19 was a multi-centre trial that recruited 230 patients without known CAD, referred for invasive angiography. The prevalence of obstructive disease in the studied population was 13.9%. The sensitivity and specificity of CTCA for detecting obstructive (>70% stenosis) coronary disease was 83% and 83%, respectively. Importantly, the negative predictive value (NPV) was 99%. It should be noted, however, that the positive predictive value (PPV) was only 48%. The PPV of CTCA is increased if it is performed in populations with higher disease prevalence, or if the threshold for diagnosing obstructive stenosis is reduced to 50%. In a study of 360 patients evaluating CTCA in a population with a high prevalence of obstructive disease (68%), Meijboom et al. found a sensitivity, specificity, NPV and PPV to detect stenosis of >50% of 99%, 64%, 97% and 86%, respectively.20
These studies demonstrate the usefulness of CTCA to exclude the diagnosis of obstructive disease in populations with suspected CAD. However, they also demonstrate only moderate PPV, a problem attributable to a comparatively high rate of false positives reported with CTCA. CTCA is of limited value in patients at very low risk of CAD, not least because PPV is reduced in populations with very low disease prevalence. Conversely, it does not add to the management of high-risk patients who will already be undergoing intensive medical therapy, and who will still require invasive angiography if revascularisation is being considered. Recent consensus guidelines12 suggest that CTCA is an appropriate investigation to:
- Evaluate patients with stable chest pain and an intermediate pre-test probability of CAD with an un-interpretable or equivocal stress test.
- In acute chest pain with an intermediate pre-test probability of CAD and no ECG or enzyme changes suggestive of acute coronary syndrome (ACS).
- To evaluate congenital coronary abnormalities.
- To assess the aetiology of new-onset heart failure.
Atherosclerotic plaque imaging using CT
Conventional angiography visualises only the arterial lumen. A potential advantage of CT is the ability to highlight plaque within the wall. Quantification of this plaque provides a truer portrait of the extent of coronary disease as it will include plaque in arteries with ‘positive remodelling’, where the arterial segment has expanded outward to accommodate the plaque with minimal lumen loss. The composition of plaque is more important for risk stratification than the stenosis it causes; the majority (approximately two-thirds) of MIs result from disruption of plaques causing less than 50% stenosis.21 In broad terms, cardiac CT can detect three different types of coronary plaque; calcified, non-calcified and mixed (elements of both).
Atherosclerosis imaging requires contrast-enhanced scans with the highest possible spatial resolution. A study comparing 64-slice CT with intravascular ultrasound (IVUS) reported that CTCA correctly identified 95% of calcified plaques, 83% of non-calcified plaques and 94% of mixed plaques.22 Plaque volume estimation by CT shows moderate correlation with IVUS, however, inter-observer variability is high.23,24 Over-estimating calcified plaque volume is a common problem with CT as the attenuation of calcium is so much greater than other structures – this results in ‘partial voluming’.25
Prognostically, the presence of plaque of any degree is important. In a prospective study of 100 patients, there was a higher cardiovascular event rate at 16 months in those with demonstrated coronary plaque compared with those with no disease, even if the plaque was non-obstructive.26 Interestingly, the prevalence of non-calcified plaque is higher in the culprit lesions of patients with ACS than those with stable angina.27
CT for the detection of vulnerable plaque
Analysis of post-mortem studies reveals that plaque rupture is the precipitating event in approximately two-thirds of cases of fatal coronary thrombosis.22 Plaques at risk of rupture have a specific morphology – the ‘thin-capped fibroatheroma’ (TCFA), with large, lipid-rich necrotic cores and thin overlying fibrous caps (<65 µm).28 Aggressive medical treatment has been shown to reduce clinical events in some patients that do not score highly using Framingham scores.29 In view of this, there is a requirement for a non-invasive modality that can detect high-risk coronary plaques to allow intensive treatment of potentially vulnerable patients. Pundziute et al. performed 64-slice CT and virtual histology IVUS (VH-IVUS) in patients with ACS or stable angina. They found that non-calcified plaque and mixed plaque were more prevalent in ACS patients, while calcified plaques were more prevalent in stable patients. They also found that VH-IVUS-defined TCFAs were more common in patients with ACS than stable angina, and that the TCFAs most frequently occurred in plaques classified as mixed by CT.30 Attempts to identify TCFA using CT alone are hampered by technical challenges. The maximum spatial resolution of modern CT scanners is 330 µm. Given that the fibrous cap of the TCFA is by definition less than 65 µm in diameter, we have to accept that it will not be possible to image thin fibrous caps by CT. Lipid-rich necrotic core detection is more feasible appearing as areas of low attenuation on CT (figure 3).
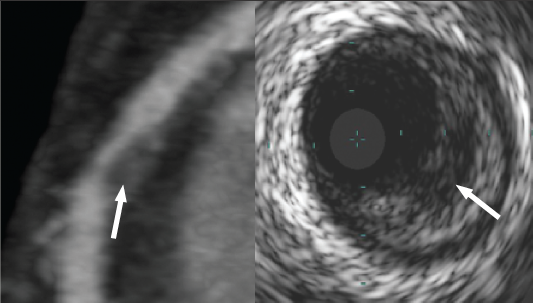
Work using phantoms has shown that it is theoretically possible to differentiate soft (lipid) and intermediate (fibrous) components of non-calcified plaque on the basis of their attenuation values.31,32 Studies comparing CT with IVUS and post-mortem histology have confirmed that lipid-rich plaque has lower attenuation than fibrous plaque.33,34 However, its utility is limited by overlap of the two attenuation ranges, and by the fact that lipid cores may be beyond the spatial resolution of CT. This can be overcome by imaging only the larger proximal coronary segments, which in one study allowed identification of 70% of lipid pools compared with IVUS.35 There are two other features of plaque vulnerability detectable by CT, namely positive remodelling36 and spotty calcification37 (figure 4).
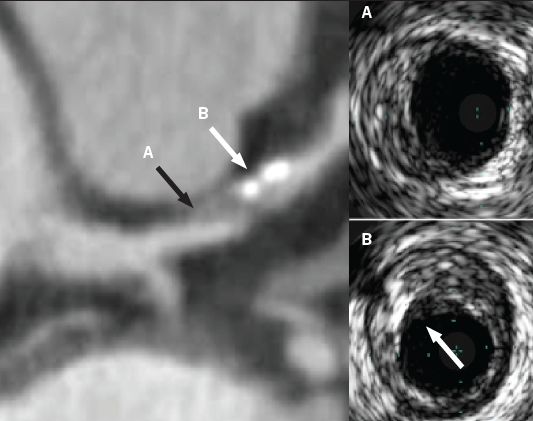
A CT study in patients with either ACS or stable angina documented the presence of low attenuation plaque (<30 HU) as well as positive remodelling and spotty calcification. All three high-risk features were significantly more common in the arteries of those with ACS than stable symptoms. In addition, the presence of all three features yielded a PPV of 95% that the plaque was associated with an ACS, and the absence of all three features had a NPV of 100%.38 Importantly, this group of investigators have used the same CT features to perform a prospective study of over 1,000 patients followed for two years. They found that both positive arterial remodelling and plaques with attenuation values <30 HU were both independently associated with subsequent ACS, and that the presence of both together gave a hazard ratio of 23.39
Future developments
The field of cardiac CT continues to develop at a fast pace. Recent innovations include 320-detector machines that can image the heart in a single beat, and dual-source imaging with two X-ray sources and two detector arrays5 delivering superior temporal resolution.40,41 There has been concern recently regarding the dose of radiation attributed to cardiac CT.42 The radiation dose of a CTCA examination varies, depending on the patient, scanner, and protocol used. From early reports of doses in excess of 20 mSv,12 multiple dose-reduction strategies have been devised including reduced tube voltage,43 tube current modulation,44 very high pitch imaging45 and prospective gating.46 A recent series using dual-source CT with high pitch reported diagnostic images at less than 1 mSv.45
The ability of CT to classify coronary plaque opens up the possibility of better risk prediction. There is also potential for serial plaque imaging to track the effect of drugs on coronary atherosclerosis. Sequential CAC scores have proven unreliable in detecting plaque regression to date, with a large placebo-controlled trial showing no decrease with statin therapy, despite a significant reduction in low-density lipoprotein (LDL)-cholesterol.47 It has been suggested that a more accurate analysis of plaque progression/regression can be achieved by quantification of all plaque types and not just calcified plaque.48 Classifying non-calcified plaque more precisely could potentially detect ‘vulnerable plaque’. With current scanner technology a dramatic increase in spatial resolution is unlikely in the near future, however, there are other potential avenues to improve detection of vulnerable plaque. These include the use of multiple energy data sets to reduce the overlap of the attenuation of plaque components, which would improve their classification,17 and the possibility of new contrast agents to target inflammatory components, such as macrophages, to highlight areas of potential vulnerability.49
Acknowledgements
Work described in this manuscript was part-funded by the NIHR Cambridge Biomedical Research Centre. Dr D R Obaid is the recipient of a Sackler award.
Conflict of interest
None declared.
Editors’ note
This article follows previous articles in this series on IVUS-derived virtual histology (2010;17:129–32) and optical coherence tomography (2010;17:190–3). See also the editorial by Alfakih and Budoff regarding the radiation burden associated with MDCT on pages 207-08 of this issue.
Key messages
- Indiscriminate use of calcium scoring or computed tomography (CT) angiography in asymptomatic patients as a screening tool for coronary disease is not supported
- Calcium scoring can provide prognostic information in selected patients above that obtained from conventional risk factors
- CT angiography can be useful to exclude significant coronary disease in symptomatic patients at low-to-intermediate risk
- Cardiac CT visualises coronary plaque, as well as lumen, and may provide the opportunity to detect ‘vulnerable’ plaque in the future
References
1. Hounsfield GN. Computerized transverse axial scanning (tomography). 1. Description of system. Br J Radiol 1973;46:1016–22.
2. Hounsfield GN. The E.M.I. scanner. Proc R Soc Lond B Biol Sci 1977;195:281–9.
3. Achenbach S, Ropers D, Holle J, Muschiol G, Daniel WG, Moshage W. In-plane coronary arterial motion velocity: measurement with electron-beam CT. Radiology 2000;216:457–63.
4. Sagel SS, Weiss ES, Gillard RG et al. Gated computed tomography of the human heart. Invest Radiol 1977;12:563–6.
5. Flohr TG, McCollough CH, Bruder H et al. First performance evaluation of a dual-source CT (DSCT) system. Eur Radiol 2006;16:256–68.
6. Boyd DP, Lipton MJ. Cardiac computed tomography. Proceedings of the IEEE 1982;71:298–307.
7. Agatston AS, Janowitz WR, Hildner FJ et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–32.
8. Stary HC. Composition and classification of human atherosclerotic lesions. Virchows Arch A Pathol Anat Histopathol 1992;421:277–90.
9. Beckman JA, Ganz J, Creager MA, Ganz P, Kinlay S. Relationship of clinical presentation and calcification of culprit coronary artery stenoses. Arterioscler Thromb Vasc Biol 2001;21:1618–22.
10. Budoff MJ, Shaw LJ, Liu ST et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol 2007;49:1860–70.
11. Greenland P, Bonow RO, Brindage BH et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol 2007;49:378–402.
12. Schroeder S, Achenbach S, Bengel F et al. Cardiac computed tomography: indications, applications, limitations, and training requirements: report of a Writing Group deployed by the Working Group Nuclear Cardiology and Cardiac CT of the European Society of Cardiology and the European Council of Nuclear Cardiology. Eur Heart J 2008;29:531–56.
13. National Institute for Health and Clinical Excellence. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. (Final draft). London: NICE, 2010.
14. Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation 1995;92:2157–62.
15. Rosen BD, Fernandes V, McClelland RL et al. Relationship between baseline coronary calcium score and demonstration of coronary artery stenoses during follow-up MESA (Multi-Ethnic Study of Atherosclerosis). JACC Cardiovasc Imaging 2009;2:1175–83.
16. Patel MR, Peterson ED, Dai D et al. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010;362:886–95.
17. Halliburton SS. Recent technologic advances in multi-detector row cardiac CT. Cardiol Clin 2009;27:655–64.
18. Sirol M, Sanz J, Henry P, Rymer R, Leber A. Evaluation of 64-slice MDCT in the real world of cardiology: a comparison with conventional coronary angiography. Arch Cardiovasc Dis 2009;102:433–9.
19. Budoff MJ, Dowe D, Jolly JG et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease. J Am Coll Cardiol 2008;52:1724–32.
20. Meijboom WB, Meijs MF, Schuijf JD et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008;52:2135–44.
21. Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation 1995;92:657–71.
22. Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol 2006;47(8 Suppl):C13–C18.
23. Achenbach S, Moselewski F, Ropers D et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation 2004;109:14–17.
24. Moselewski F, Ropers D, Pohle K et al. Comparison of measurement of cross-sectional coronary atherosclerotic plaque and vessel areas by 16-slice multidetector computed tomography versus intravascular ultrasound. Am J Cardiol 2004;94:1294–7.
25. Schoenhagen P, Barreto M, Halliburton SS. Quantitative plaque characterization with coronary CT angiography (CTA): current challenges and future application in atherosclerosis trials and clinical risk assessment. Int J Cardiovasc Imaging 2008;24:313–16.
26. Pundziute G, Schuijf JD, Jukema JW et al. Prognostic value of multislice computed tomography coronary angiography in patients with known or suspected coronary artery disease. J Am Coll Cardiol 2007;49:62–70.
27. Hoffmann U, Moslewski F, Nieman K et al. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol 2006;47:1655–62.
28. Kolodgie FD, Burke AP, Farb A et al. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol 2001;16:285–92.
29. Ridker PM, Danielson E, Fonseca FA et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–207.
30. Pundziute G, Schuijf JD, Jukema JW et al. Evaluation of plaque characteristics in acute coronary syndromes: non-invasive assessment with multi-slice computed tomography and invasive evaluation with intravascular ultrasound radiofrequency data analysis. Eur Heart J 2008;29:2373–81.
31. Horiguchi J, Fujioka C, Kiguchi M et al. Soft and intermediate plaques in coronary arteries: how accurately can we measure CT attenuation using 64-MDCT? AJR Am J Roentgenol 2007;189:981–8.
32. Schroeder S, Flohr T, Kopp AF et al. Accuracy of density measurements within plaques located in artificial coronary arteries by X-ray multislice CT: results of a phantom study. J Comput Assist Tomogr 2001;25:900–06.
33. Schroeder S, Kuettner A, Leitritz M et al. Reliability of differentiating human coronary plaque morphology using contrast-enhanced multislice spiral computed tomography: a comparison with histology. J Comput Assist Tomogr 2004;28:449–54.
34. Pohle K, Achenbach S, Macneill B et al. Characterization of non-calcified coronary atherosclerotic plaque by multi-detector row CT: comparison to IVUS. Atherosclerosis 2007;190:174–80.
35. Leber AW, Becker A, Knez A et al. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J Am Coll Cardiol 2006;47:672–7.
36. Schoenhagen P, Ziada KM, Kapadia SR, Crowe TD, Nissen SE, Tuzcu EM. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation 2000;101:598–603.
37. Ehara S, Kobayashi Y, Yoshiyama M et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation 2004;110:3424–9.
38. Motoyama S, Kondo T, Sarai M et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 2007;50:319–26.
39. Motoyama S, Sarai M, Harigaya H et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009;54:49–57.
40. Ropers U, Ropers D, Pflederer T et al. Influence of heart rate on the diagnostic accuracy of dual-source computed tomography coronary angiography. J Am Coll Cardiol 2007;50:2393–8.
41. Alkadhi H, Scheffel H, Desbiolles L et al. Dual-source computed tomography coronary angiography: influence of obesity, calcium load, and heart rate on diagnostic accuracy. Eur Heart J 2008;29:766–76.
42. Fazel R, Krumholz HM, Wang Y et al. Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med 2009;361:849–57.
43. Bischoff B, Hein F, Meyer T et al. Impact of a reduced tube voltage on CT angiography and radiation dose: results of the PROTECTION I study. JACC Cardiovasc Imaging 2009;2:940–6.
44. Herzog P, Jakobs TF, Wintersperger BJ, Nikolaou K, Becker CR, Reiser MF. [Radiation dose and dose reduction in multidetector row CT (MDCT)]. Radiologe 2002;42:691–6.
45. Achenbach S, Marwan M, Ropers D et al. Coronary computed tomography angiography with a consistent dose below 1 mSv using prospectively electrocardiogram-triggered high-pitch spiral acquisition. Eur Heart J 2010;31:340–6.
46. Hsieh J, Londt J, Vass M et al. Step-and-shoot data acquisition and reconstruction for cardiac x-ray computed tomography. Med Phys 2006;33:4236–48.
47. Raggi P, Davidson M, Callister TQ et al. Aggressive versus moderate lipid-lowering therapy in hypercholesterolemic postmenopausal women: Beyond Endorsed Lipid Lowering with EBT Scanning (BELLES). Circulation 2005;112:563–71.
48. Lehman SJ, Schlett CL, Bamberg F et al. Assessment of coronary plaque progression in coronary computed tomography angiography using a semiquantitative score. JACC Cardiovasc Imaging 2009;2:1262–70.
49. Hyafil F, Cornily JC, Feig JE et al. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med 2007;13:636–41.
