Ictal bradycardia/asystole is a poorly recognised cause of collapse late in the course of a typical complex partial seizure. Its recognition is important as it might potentially lead to sudden unexpected death in epilepsy (SUDEP). We present five patients with intractable complex partial seizures who had associated ictal bradycardia/asystole. All the patients underwent cardiac pacing to potentially prevent SUDEP. It is important to recognise and treat ictal asystole early, and to achieve this there is need for both an increase in epilepsy monitoring beds and a recognition of the potential role of implantable loop recorders in the evaluation of patients with epilepsy who clinically appear to be at increased risk for ictal asystole.
Introduction
Heart rhythm changes are common during seizures, even those seizures not associated with convulsive activity. Most studies report tachycardia, a heart rate increase of more than 10 beats per minute above the baseline, as the most common rhythm abnormality occurring in 64–100% of temporal lobe seizures.1,2 By contrast, ictal bradycardia has been reported in less than 6% of patients with complex partial seizures.3,4 The ictal bradycardia syndrome occurs in those with established epilepsy when epileptic discharges disrupt normal cardiac rhythm leading to a decrease in heart rate of more than 10 beats per minute below the baseline. The majority of patients with ictal bradycardia have temporal lobe seizures. It is believed that abnormal neuronal activity during a seizure can affect central autonomic regulatory centres in the brain leading to cardiac rhythm changes. It is important to identify ictal bradycardia as a potential harbinger of lethal rhythms, such as asystole, as this may be one important mechanism leading to sudden unexpected death in epilepsy (SUDEP).5 Ictal bradycardia/asystole may be unrecognised until documented during video-electroencephalograph (EEG)–electrocardiogram (ECG) monitoring in those with refractory epilepsy, often in the context of pre-surgical evaluation. Here we present five patients with intractable complex partial seizures associated with ictal bradycardia or asystole. All the patients underwent cardiac pacing to potentially prevent SUDEP.
Patient 1
This is a 50-year-old right-handed man who has had focal epilepsy since the age of 25 years. He described his attacks as an unusual sensation over his right shoulder, or simply “a presence” followed by heat rising through the right side of his body. He then experienced a heightened feeling that something was about to happen. This was followed by loss of awareness, pulling at his clothes, facial grimacing and talking incoherently. Each of these episodes lasted about 60 seconds. He recovered quickly after each event. Occasionally the attacks were more severe resulting in falls and injury. He usually experienced an urge to micturate after a seizure. The seizure frequency was one every fortnight. He had no early risk factors for epilepsy. Neurological examination was normal. Inter-ictal EEG showed epileptic discharges in the left fronto-temporal region.
Magnetic resonance imaging (MRI) of the brain showed an area of high signal in the left posterior parietal lobe, which was felt to represent either a low grade glioma or an area of cortical dysplasia. The patient was admitted to the monitoring unit for video-EEG–ECG telemetry as part of the work up for epilepsy surgery (figure 1). At the time of admission, the patient was taking slow-release carbamazepine 400 mg twice daily, slow-release sodium valproate 1,000 mg twice daily and levetiracetam 1,500 mg twice daily. The patient had two seizures while being monitored, both complex partial seizures with electrographic onset from the left temporal region. One of the seizures was associated with ictal asystole. The heart rate at seizure onset was 60 beats per minute, slowing down to 55 beats per minute, followed by a 96 second period of asystole. The period of bradycardia and asystole was preceded by the epileptic seizure. The patient underwent cardiac pacing. No further seizures associated with falls have occurred.
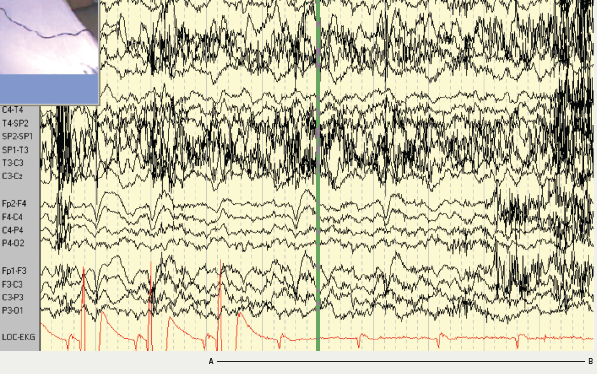
Patient 2
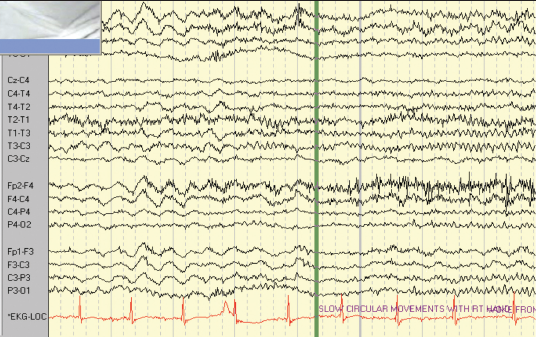
The second patient is a 47-year-old right-handed woman with seizure onset from the age of seven years. Her seizures were characterised by loss of awareness, associated with “fumbling” with her clothes. The seizures were occasional secondarily generalised. She sometimes experienced palpitations and felt she was “going insane”. Seizure frequency at the time of monitoring was two to three per week. She had a normal neurological examination. Her anti-epileptic medication included slow-release carbamazepine 400 mg twice daily and phenytoin 125 mg twice daily. The patient had previously had a vagal nerve stimulator inserted to try and reduce the seizure frequency, but it had little impact on her seizures. MRI showed a lesion in the right temporal lobe consistent with either a low grade glioma or a dysembryoplastic neuroepithelial tumour (DNET). The patient had video-EEG–ECG monitoring over an eight-day period and a total of six complex partial seizures and two auras were recorded (figures 2 and 3). The events were associated with bilateral automatisms, alteration of awareness and automatic wandering. Some events were associated with confused ictal speech. The patient did not exhibit loss of tone during any of the recorded seizures. The ictal EEG showed that the patient had multifocal epilepsy with seizures arising from both temporal lobes. Each of the complex partial seizures was associated with ictal asystole of 10 seconds duration on average. The patient had a cardiac pacemaker inserted.
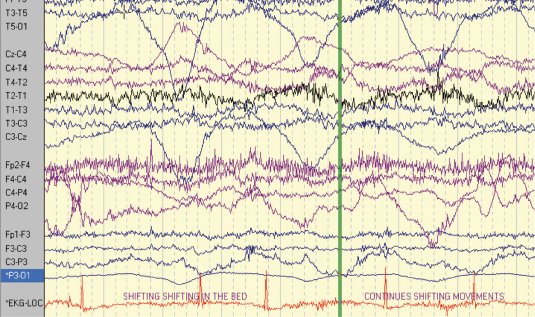
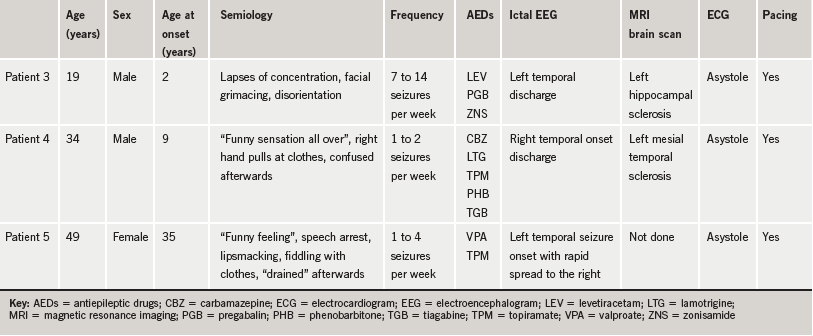
Three other patients who had asystole are tabulated below (table 1).
Discussion
The patients presented here underscore the importance of awareness of ictal bradycardia/asystole. An important distinction is to be made between ictal asystole and convulsive syncope. In ictal asystole, the primary event is the seizure. The epileptic discharge then leads to disruption of the normal cardiac rhythm resulting in slowing of the heart rate or asystole. Clinically this manifests as unexpected collapse or fall late in the course of a typical complex partial seizure. On the other hand, syncope is abrupt loss of consciousness due to a sudden drop in cerebral perfusion. The cause is often relatively benign vasovagal or neurocardiogenic syncope, which results from excessive vagal tone. Vasovagal syncope is usually associated with a precipitant such as standing or venepuncture. Syncope may, however, be caused by potentially fatal cardiac arrhythmias. In convulsive syncope, short-lived myoclonic jerks, tonic spasms, salivation or multifocal jerks may accompany syncope if recovery from cerebral hypoperfusion is delayed. This occurs usually if the head is held in an upright position. The term secondary anoxic seizure is sometimes used. It is not a true seizure and is not associated with epileptic EEG changes. Ictal asystole usually occurs in the context of refractory complex partial seizures while convulsive syncope usually occurs in individuals without epilepsy.6
In ictal asystole, there should be clear evidence of an initial seizure. It is important to differentiate between neurocardiogenic syncope and ictal asystole for two reasons; first, ictal asystole is potentially life threatening as it may be a significant risk factor for SUDEP.7,8 Cardiac pacemaker insertion is, therefore, advised in patients with documented ictal asystole and would be the practice of most epilepsy centres. On the other hand, patients with syncope, if inappropriately treated may be adversely affected by anti-epileptic medication, such as carbamazepine, which may prolong the QT interval and, thereby, potentially cause cardiac arrhythmias. Patients with intractable focal epilepsy are at a higher risk for ictal asystole, while generalised tonic-clonic seizures have been associated with an increased risk for SUDEP. Many of these tonic-clonic seizures have partial onset with subsequent secondary generalisation.9,10 The diagnostic yield of ictal asystole is increased by prolonged video-EEG–ECG monitoring.11 The scarcity of epilepsy monitoring facilities means that ictal asystole will probably remain under-recognised. A possible way of increasing the diagnostic yield of ictal asystole is the use of implantable loop recorders (ILR). The implantable loop recorder can provide continuous long-term ECG home monitoring, and is particularly useful for investigating patients with infrequent symptoms.12 Patients may be monitored for up to one year without the need for hospital admission. Patients with temporal lobe epilepsy who present with unexpected collapse, loss of tone or falls late in the course of a typical complex partial seizure are clinically in a high-risk category for ictal asystole as delayed loss of tone is uncommon in temporal lobe epilepsy.13
Intuitively, we postulate that early diagnosis and treatment of ictal asystole in patients with refractory epilepsy could prevent SUDEP. To achieve this, we recommend that patients with temporal lobe seizures who present with collapse or loss of tone late in the course of a complex partial seizure should either be admitted for video-EEG–ECG monitoring or referred to a cardiologist for an ILR.12 This will facilitate early diagnosis of ictal asystole and treatment by cardiac pacing. It is also apparent that there needs to be an increase in the number of epilepsy monitoring beds.
Conflict of interest
None declared.
Editors’ note
A review article looking at ‘Epilepsy and the heart’ by Drs F Rugg-Gunn and D Holright can be found on pages 233–9 of this issue.
References
1. Marshall DW, Westmoreland BF, Sharbrough FW. Ictal tachycardia during temporal lobe seizures. Mayo Clinic Proc 1983;58:443–6.
2. Blumhardt LD, Smith PE, Owen L. Electrocardiographic accompaniments of temporal lobe epileptic seizures. Lancet 1986;1:1051–6.
3. Smith PE, Howell SJ, Owen L, Blumhardt LD. Profiles of instant heart rate during partial seizures. Electroencephalogr Clin Neurophysiol 1989;72:207–17.
4. Nei M, Ho RT, Sperling MR. EKG abnormalities during partial seizures in refractory epilepsy. Epilepsia 2000;41:542–8.
5. Oppenheimer SM, Cechetto DF, Hachinski VC. Cerebrogenic cardiac arrhythmias: cerebral electrocardiographic influences and their role in sudden death. Arch Neurol 1990;47:513–19.
6. McKeon A, Vaughan C, Delanty N. Seizures versus syncope. Lancet Neurol 2006;5:171–80.
7. Bergen DC. In a heartbeat: autonomic changes during seizures. Epilepsy Curr 2005;5:194–6.
8. Hirsh LJ, Hauser WA. Can sudden unexplained death in epilepsy be prevented? Lancet 2004;364:2157–8.
9. Nashef L, Garner S, Sander JW, Fish DR, Shorvon SD. Circumstances of death in sudden death in epilepsy: interviews of bereaved relatives. J Neurol Neurosurg Psychiatry 1998;64:349–52.
10. Langan Y, Nashef L, Sander JW. Sudden unexpected death in epilepsy: a series of witnessed deaths. J Neurol Neurosurg Psychiatry 2000;68:211–13.
11. Britton JW, Ghearing RG, Benarroch EE, Cascino GD. The ictal bradycardia syndrome: localisation and lateralisation. Epilepsia 2006;47:737–44.
12. Rugg-Gunn FJ, Simister RJ, Squirrell M, Holdright DR,
Duncan JS. Cardiac arrhythmia
in focal epilepsy: a prospective long term study. Lancet 2004;364:2212–19.
13. Schuele SU, Alexopoulos AV, Locatelli ER, Dinner DS. Video-electrographic and clinical features in patients with ictal asystole. Neurology 2007;69:434–41.
