The outcome and complications of atrial fibrillation (AF) ablation in a UK patient cohort were investigated by offering symptomatic, drug-refractory patients ablation. Treatment goals were to disconnect all pulmonary veins electrically and improve symptoms using a state-of-the-art ablation method. Outcomes were defined as: ‘success’ (no symptoms or Holter AF); ‘partial success’ (substantially reduced AF symptoms); ‘clinical success’ (‘success’ and ‘partial success’); ‘failure’ (no symptom improvement).
A total of 100 consecutive patients (age: 49 years [range 37–76]; females: n=17; persistent AF: n=30; CHADS2 score >1: n=7) underwent a first ablation (between January 2004 and May 2007). Ultimately 167 procedures were performed until follow-up censure in May 2009. Complications occurred in 15 patients – acutely in 11, during follow-up in four. Cumulative ‘success’, ‘partial success’, ‘failure’ and ‘clinical success’ rates after 22 ± 14 months were 60%, 26%, 14% and 86%, respectively. ‘Clinical success’ rates for paroxysmal and persistent subgroups were 73% and 47% (first procedure) rising to 87% and 83% (all procedures). Numbers of patients needing anti-arrhythmic drugs reduced significantly (p<0.0001).
In conclusion, catheter ablation improves symptoms in 83–87% of patients, reduces objective AF burden and the need for anti-arrhythmic drug therapy. It should be recommended routinely to symptomatic patients with drug-refractory AF without demonstrable heart disease.
Introduction
Catheter-ablation is an established treatment for patients with uncontrolled symptoms of atrial fibrillation (AF).1 However, many cardiologists remain sceptical about its efficacy, and clinicians in general cannot relate ablation to the majority of AF patients.2-4 Although ablation techniques vary, there is general consensus that it is crucial to treat all pulmonary vein–left atrial junctions.5,6 Outcomes have usually been reported in terms of absolute success or failure after a short time period – not easily reconcilable with patient experiences subsequently.7 Recently, large amalgamated experiences have been published, which help explain some earlier inconsistencies, but these assume uniformity of patient selection, follow-up and ablation techniques.8
Unlike in other arrhythmias, AF presents ‘a moving target’ for ablation.9-11 As arrhythmia burden increases, ever more extensive ablation may be required to control it.12 In patients with persistent AF, ablation results remain tantalisingly unpredictable, even after extensive bi-atrial procedures.13-18 Similarly, patients with paroxysmal AF can be at different stages of atrial remodelling and do not respond uniformly to pulmonary vein isolation alone.19-21
The aim of this analysis was to report the AF ablation patient journey, based on a detailed evaluation of the course and outcome of 100 consecutive patients, treated in a uniform way by experienced operators at a single UK tertiary referral hospital. The object is to help clinicians better understand the nuances of AF ablation outcomes, largely concealed in larger series.
Methods
Patient selection
Patients with symptomatic AF without demonstrable heart disease, who were inadequately controlled by drug therapy, were considered for ablation. Structural heart disease and myocardial ischaemia were excluded as previously described.7 All were motivated to undergo ablation by intolerable symptoms or lack of acceptance of AF.
Ablation procedure
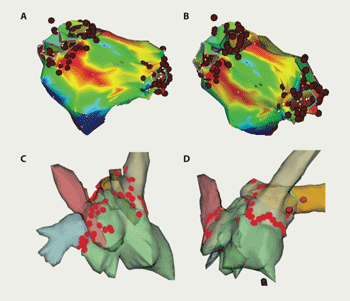
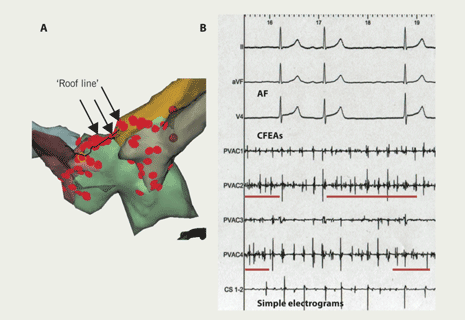
Ablation was performed in a uniform way. The left atrium was accessed by double-wiring of a single trans-septal puncture. An internal cardioversion/pacing catheter was positioned in the coronary sinus and a Lasso catheter (Biosense Webster Inc., CA, USA) used to guide ablation and confirm electrical isolation of veins. Ablation was performed using irrigation-tipped Webster 4 mm (Celsius ‘Thermocool’, Biosense Webster) or Navistar 4 mm catheters (Biosense Webster Inc., CA, USA). Patients were anticoagulated with unfractionated heparin, to maintain an activated clotting time longer than 250 seconds. Navigation and ablation were guided by either NavX-ESI (St Jude Medical Inc., St Paul, USA) or CARTO-XP (Biosense Webster Inc., CA, USA) mapping systems. Ablation lesions were sited around each pulmonary vein separately (figure 1). The acute procedural goal was to isolate all identified pulmonary veins (PVI). Ablation energy was power limited to 30 W, temperature to 50 ºC with irrigation flow rate of 17–30 ml/min. Additional linear lesions (figure 2A) were performed in patients with persistent AF or in any who continued in AF despite PVI. Focal sites at which complex fractionated electrograms were consistently recorded during AF – possible perpetuators of the arrhythmia – were also targeted for specific ablation at repeat procedures17,18,22 (figure 2B). Afterwards, patients with a history of long paroxysms or persistent AF continued their anti-arrhythmic drugs. All were on either warfarin or aspirin.
Follow-up
Patients were reviewed regularly out to 12 months following each procedure, their rhythm status determined on the basis of symptoms and a 12-lead electrocardiogram (ECG) at each visit. Only arrhythmias continuing beyond a two-month ‘blanking period’ are reported. All patients, free of AF symptoms, also had 48-hour to seven-day Holter ECG recordings. Anti-arrhythmic therapy was withdrawn three months after ablation. However, if symptoms returned later, therapy was restarted and the patient offered repeat ablation. Most patients who continued to experience symptoms despite anti-arrhythmic therapy opted for repeat ablation(s). Three outcome categories were defined: ‘success’ (i.e. no symptoms, ECG or Holter evidence of AF lasting >30 seconds; no class 1C or III anti-arrhythmic therapy); ‘partial success’ (i.e. AF symptoms and objective AF burden substantially reduced on or off previously ineffective, anti-arrhythmic therapy); ‘failure’ (i.e. no clinically relevant change in symptoms or AF burden). Follow-up was censured on 31st May 2009.
Patients’ own assessment of ablation outcome
An anonymised questionnaire was distributed by the hospital’s Clinical Governance Department in spring 2008 to assess:
- symptom impact on quality of life pre-ablation
- effect of ablation outcome on symptoms and quality of life
- whether they would undergo ablation treatment again, or recommend it to others.
Results were correlated with objective arrhythmia outcomes to derive the fourth outcome category – ‘clinical success’ (i.e. the sum of ‘success’ and ‘partial success’ categories (clinician assessed) combined with patients’ own evaluation).
Statistical methods
All values are expressed as mean and one standard deviation. Comparisons between groups were made using Student’s t and Fishers’ exact testing. Subgroup comparisons were made using analysis of variance. Differences were considered significant at the 5% level.
Results
By 31st May 2009, over 1,000 AF ablation procedures had been performed at this hospital, using a variety of techniques.7 The 100 consecutive patients reported in detail here were those without demonstrable heart disease, who had a first procedure for AF in the period between June 2004 and June 2007 using a uniform, ‘state-of-the-art’ ablation method. Mean age was 55 ± 11 years (range 22–74), length of AF history was 66.5 months (range 3–240), 83 were male and 30 (30%) had persistent AF. In only seven was CHADS2 risk score greater than one (CHADS2 scores: 0 n=69; 1 n=24; 2 n=3; 3 n=3; 4 n=1). Scores were contributed to by the following: heart failure at presentation due to tachycardia myopathy (n=4), treated hypertension (n=25), age over 75 years (n=1), diabetes mellitus (n=5) and stroke (n=4). No patient had prior infarction, active myocardial ischaemia or idiopathic cardiomyopathy. AF symptoms had not been controlled by potent anti-arrhythmic drugs. Fifty-six had failed flecainide or propafenone and 13 had failed amiodarone or sotalol in high dose. Most patients were also taking beta-blocking agents, calcium channel blockers or digoxin prior to their first ablation.
Cumulative outcome results
In total, 167 separate ablation procedures were performed in these 100 patients and procedure-related complications occurred in 15 (15%) (tables 1 and 2). Acute complications included cardiac tamponade, requiring pericardiocentesis in five (3% of procedures), pericarditis with effusion treated conservatively in five (3% of procedures), significant discomfort at catheter access site in two (1%) and allergic iodine contrast reaction in one (0.6% of procedures).
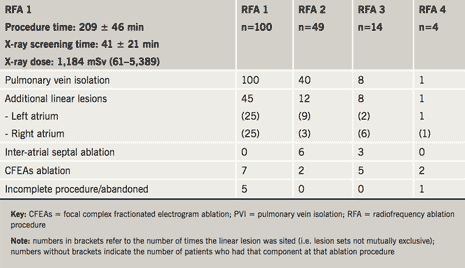
Late complications occurred in four (4%) patients – one (0.6% of procedures) each of – unexplained sudden death six months after ablation, minor stroke with normal brain imaging, pulmonary vein stenosis of more than 75% (treated conservatively), pulmonary embolus 10 days after ablation (managed conservatively). Left atrial flutter occurred in eight (8%) patients and was typically persistent and more symptomatic than AF. After a mean follow-up of 22 months (range 3–92 months) cumulative ‘success’, ‘partial success’ and ‘failure’, as defined, for the whole group were 60%, 26% and 14%, respectively. Outcomes for paroxysmal AF at time of first ablation were 61%, 26% and 13%, respectively, after 1.5 ± 0.7 procedures per patient and for persistent AF, 57%, 26% and 17%, respectively, after 2 ± 1 procedures (‘success’: p=0.66).
Patients’ own assessment of ablation outcome
Of the 100 patient questionnaires circulated 12 months after ablation, 80 were returned and analysed. Sixty-five (81%) described symptoms as “bad” or “terrible” prior to ablation. Of these, 58 (89%) felt “much better” afterwards – symptoms having improved to “none” or “not bad at all” (p<0.0001). Seventy-four (92%) described AF as interfering with their life at least “modestly” prior to ablation. Afterwards, 40 (58%) felt symptoms did not affect their quality of life “at all” (p<0.001), 29 (42%) rated interference as “only a little” and 11 (14%) as “moderately interfering” with their life. Seventy-four (92%) indicated that ablation had improved symptoms sufficiently for them to undergo the procedure, if faced with the same choice again, four were unsure and two said that they would not have opted for ablation. These results support the clinical impression that the outcome ‘partial (anti-arrhythmic) success’, as defined, also indicates patients who, although not free of AF, have benefited substantially from the procedure. Therefore, defining ‘clinical success’ as the sum of objective clinician-defined ‘success’ and ‘partial success’ indicates those who have either no AF or are sufficiently improved, in their own estimation, not to need further intervention. ‘Clinical success’ was achieved in 86% of the 100 patients (paroxysmal AF 87%; persistent AF 83%; p=0.75).
Does ‘failure’ of first AF ablation indicate a non-responder or the need for a repeat procedure?
As indicated by the following analysis, cumulative benefits accrue from repeat ablation procedures (table 2).
First ablation procedure
Although all identified pulmonary vein–left atrial junctions were treated, complete electrical isolation could only be confirmed in 77 (77%). Complications occurred in 10 (10%) patients. Overall ‘clinical success’ rate was 67%, but ‘success’ and ‘clinical success’ rates were significantly better for paroxysmal than for persistent AF (‘success’: 46% versus 16%, p=0.007; ‘clinical success’: 77% versus 43%, p=0.002).
Second ablation procedure
All patients continuing to experience AF symptoms were recommended repeat ablation and 49 had a second procedure 12.7 ± 8.1 months after the first. Pulmonary vein reconnections were found in some or all veins in 40 (82%) patients and complications occurred acutely in four (8%). There were no late complications. Overall ‘clinical success’ for the second procedure was 76%. Differences in ‘success’ and ‘clinical success’ rates between initially paroxysmal and persistent AF were no longer significant (‘success’: 47% versus 26%, p=0.23; ‘clinical success’: 80% versus 68%, p=0.49).
Third ablation procedure
Fourteen patients (14%) underwent a third AF ablation 26.7 ± 19.0 months after their first procedure. Pulmonary vein reconnections were found in eight (57%). One patient developed pericarditis, treated conservatively, and four developed atypical atrial flutter. Overall ‘clinical success’ for the third procedure was 71%. ‘Success’ and ‘clinical success’ rates were the same for paroxysmal and persistent AF patients (‘success’: 40% versus 22%, p=0.55; ‘clinical success’: 60% versus 78%, p=1.0).
Fourth ablation procedure
Four patients (4%) underwent a fourth ablation 29 ± 13 months after their first procedure. The indication was left atypical atrial flutter in three. Flutters terminated with addition of a left atrial ‘roof-line’ in one, with focal ablation around right lower pulmonary vein in another and with ablation at sites of complex fractionated electrograms in a third. AF arose from the right atrial appendage in the fourth patient. There were no complications. ‘Success’ rate, therefore, was 100%.
Patients changing from rhythm to rate-control management
Eight (8%) patients changed to rate-control management – four after the first and four after the second ablation procedure. They tended to be older (64 ± 9 versus 55 ± 11 years, p≤0.01). Six required complete AV-junction ablation for symptom control.
Anti-arrhythmic drug requirements before ablation and at most recent review
Prior to ablation 73 (73%) patients were taking Class 1C or III agents, alone or in combination therapy: flecainide 53 (53%), propafenone 3 (3%), disopyramide 4 (4%), high-dose sotalol 6 (6%) and amiodarone 7 (7%). At latest follow-up 22 ± 14.6 months later, only 20 (20%) still required these drugs: flecainide 14 (14%), propafenone 2 (2%) and amiodarone 4 (4%); and, in five, flecainide was only being used as a ‘pill-in-the-pocket’ regimen (p<0.0001).

Discussion
Although only a 100 consecutive patient cohort from a larger experience, this detailed analysis complements the perspective provided by larger, amalgamated AF-ablation series and specifically relates to a UK population.7,8 Its step-by-step analysis shows that outcomes are more nuanced than conveyed by earlier series, which simply reported recurrence or complete absence of AF at some arbitrary time point.23-25 The overall ‘clinical success’ rate of 86% confirms a role for ablation as a means of controlling medically refractory symptoms. The results, at mean follow-up of 22 ± 14.6 months, are consistent with those already reported previously by our group and by other authors from pioneering hospitals.7,13,26-29 On this basis, therefore, catheter ablation should be offered routinely to symptomatic patients with AF, inadequately controlled by potent anti-arrhythmic drug therapy.
The results also show that – in addition to patients who either have no AF (‘success’) or those whose arrhythmia is unchanged (‘failure’) – there is another group (‘partial success’) who benefit sufficiently to also consider their ablation successful.26 Ninety per cent of patients in this middle category reported ‘significant improvement’ after ablation – a figure similar to the 86% ‘clinical success’ rate identified by objective arrhythmia assessment. This ‘partial success’ group, inadequately discussed in most series, is readily recognisable to clinicians who, although not performing ablations themselves, routinely follow-up these patients.25
This report highlights the difficulty in achieving long-term electrical isolation of pulmonary veins with a ‘state-of-the-art’ ablation technique. Even when pulmonary vein isolation had been achieved acutely, almost all patients needing subsequent procedures had residual or re-established connections.30 If catheter ablation of AF is to be cost-effective and be made available to the majority of patients who might benefit, pulmonary vein isolation needs to be permanent, once achieved acutely. This cannot be guaranteed currently.19-21
Although ‘success’ following a first ablation was significantly better (p<0.007) for patients with paroxysmal (46%) as compared to persistent (16%) AF, eventual outcome was similar (60% versus 57%, p=0.66). Although at odds with most reports, this observation is supported by similar results from a recent, much larger series.8 It is probably explained by the fact that patients with paroxysmal or persistent AF behaviour are essentially the same, differing only in their stage of AF natural history.8,30
Complications occurred in 15 patients (9% of procedures).31 However, most were self-limiting or resolved with treatment. There were no instances of oesophageal injury, phrenic nerve palsy or pulmonary vein stenosis needing intervention.31,32 Atypical left atrial flutter – although not really a ‘complication’ – occurred in eight (8%) patients, was usually incessant, resistant to drugs and challenging to ablate.13 It was the main indication for third and fourth procedures.
This analysis reports medium-term outcome in a consecutive cohort of patients who have undergone catheter ablation, performed in a standard way, for AF at a UK tertiary referral hospital. The results are presented in a novel way with the aim of helping clinicians not involved in the delivery of this treatment, and patients, better understand what can be offered and expected. The overall conclusion, based on the outcomes achieved, is that catheter ablation should be offered routinely to all patients with symptomatic, medically refractory AF.
Conflict of interest
None declared.
Acknowledgement
Dr Bourke is part-funded by the Northumberland, Tyne & Wear Local Clinical Research Network (LCRN).
Key messages
- Over 80% of patients with structurally normal hearts respond to catheter ablation of atrial fibrillation (AF), having less symptoms and less arrhythmia burden than they would have had with optimum anti-arrhythmic drug therapy
- Catheter ablation allows withdrawal of anti-arrhythmic therapy in the majority of cases
- Catheter ablation of AF carries a small risk of acute complications, but most are readily treatable and do not have long-term sequelae
- Catheter ablation should be offered routinely to patients with symptomatic AF, inadequately controlled by trials of potent anti-arrhythmic drug therapy
References
- Fuster V, Ryden LE, Cannom DS et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Europace 2006;8:651–745.
- Testa L, Biondi-Zoccai GGL, Russo AD, Bellocci F, Andreotti F, Crea F. Rate-control versus rhythm-control in patients with atrial fibrillation: a meta analysis. Eur Heart J 2005;26:2000–06.
- The AFFIRM Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347:1825–33.
- Naccarelli G, Gonzalez M. Atrial fibrillation and the expanding role of catheter ablation: do anti-arrhythmic drugs have a future? J Cardiovasc Pharmacol 2008;52:203–09.
- Pappone C, Oreto G, Rosanio S et al. Atrial electro-anatomic remodelling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation 2001;104:2539–44.
- Gerstenfeld EP, Callans DJ, Dixit S et al. Incidence and location of focal atrial fibrillation triggers in patients undergoing repeat pulmonary vein isolation: implications for ablation strategies. J Cardiovasc Electrophysiol 2003;14:685–92.
- Bourke JP, Dunuwille A, O’Donnell D, Jamieson S, Furniss SS. Pulmonary vein ablation for idiopathic atrial fibrillation: six month outcome of first procedure in 100 consecutive patients. Heart 2005;91:51–7.
- Bhargave S, Di Biase L, Mohanty P et al. Impact and type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: results from a multi-centre study. Heart Rhythm 2009;6:1403–12.
- Haïssaguerre M, Wright M, Hocini M, Jaïs P. The substrate maintaining persistent atrial fibrillation. Circulation 2008;1:2–5.
- Calò L, Lamberti F, Loricchio ML et al. Left atrial ablation versus biatrial ablation for persistent and permanent atrial fibrillation: a prospective and randomized study. J Am Coll Cardiol 2006;47:2504–12.
- Dixit S. Evolving strategies in catheter ablation of long-standing atrial fibrillation. Circulation 2008;1:324–6.
- Allessie MA, Boyden PA, Camm AJ et al. Pathophysiology and prevention of atrial fibrillation. Circulation 2001;103:769–77.
- Haissaguerre M, Hocini M, Sanders P et al. Catheter ablation of long-lasting persistent atrial fibrillation: clinical outcome and mechanisms of subsequent arrhythmias. J Cardiovasc Electrophysiol 2005;16:1138–47.
- Vasavakul T, Khunnawat C, Ngarmukos T. A new approach to catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol 2004;43:2044–53.
- Jais P, O’Neill M, Yoshihide T et al. Stepwise catheter ablation of chronic atrial fibrillation. J Cardiovasc Electrophysiol 2006;17(suppl):S28–S36.
- Willems S, Hanno K, Rostock T et al. Substrate modification combined with pulmonary vein isolation improves outcome of catheter ablation in patients with persistent atrial fibrillation: a prospective randomised comparison. Eur Heart J 2006;27:2871–8.
- Takahashi Y, O’Neill MD, Hocini M et al. Characterization of electrograms associated with termination of chronic atrial fibrillation by catheter ablation. J Am Coll Cardiol 2008;51:1003–10.
- Oral H, Chugh A, Good E et al. Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms. Circulation 2007;115:2606–12.
- Lo L-W, Tai C-T, Lin Y-J et al. Progressive remodelling of the atrial substrate – a novel finding from consecutive voltage mapping in patients with recurrence of atrial fibrillation after catheter ablation. J Clin Electrophysiol 2007;18:258–65.
- Oakes RS, Badger TJ, Kholmovski EG et al. Detection and quantification of left atrial structural remodelling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation 2009;119:1758–67.
- Cheema A, Vasamreddy CR, Dalal D et al. Long-term single procedure efficacy of catheter ablation of atrial fibrillation. J Intervent Card Electrophysiol 2006;15:145–55.
- Nademanee K, Oketane N. The role of complex fractionated atrial electrograms in atrial fibrillation ablation: moving to the beat of a different drum. J Am Coll Cardiol 2009;53:782–9.
- Pappone C, Oreto G, Rossanio S et al. Atrial electro-anatomic remodelling after circumferential radiofrequency pulmonary vein ablation: effect of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation 2001;104:2539–44.
- Mangrum JM, Mounsey JP, Kok LC, DiMarco JP, Haines DE. Intra-cardiac echocardiography-guided, anatomically based radiofrequency ablation of focal atrial fibrillation originating from the pulmonary veins. J Am Coll Cardiol 2002;39:1964–72.
- Stabile G, Bertaglia E, Senatore G et al. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomised, controlled study. Eur Heart J 2006;27:216–21.
- Bunch TJ, Munger TM, Friedman PA et al. Substrate and procedural predictors of outcomes after catheter ablation for atrial fibrillation in patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol 2008;19:1009–14.
- Steven D, Rostock T, Lutomsky B et al. What is the real atrial fibrillation burden after catheter ablation of atrial fibrillation? A prospective rhythm analysis in pacemaker patients with continuous atrial monitoring. Eur Heart J 2008;29:1037–42.
- Earley MJ, Abrams DJR, Staniforth A, Sporton SC, Schilling RJ. Catheter ablation of permanent atrial fibrillation: medium term results. Heart 2006;92:233–8.
- Lo L-W, Tai C-T, Lin Y-J et al. Characteristics and outcome in patients receiving multiple (more than two) catheter ablation procedures for paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2008;19:150–6.
- Verma A, Kilicaslan F, Pisano E et al. Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction. Circulation 2005;112:627–35.
- Spragg D, Dalal D, Cheema A et al. Complications of catheter ablation for atrial fibrillation: incidence and predictors. J Clin Electrophysiol 2008;19:627–31.
- Cappato R, Calkins H, Chen S-A et al. Prevalence and causes of fatal outcome in catheter ablation of atrial fibrillation. J Am Coll Cardiol 2009;53:1798–803.
