PDE-5 inhibitors
Nitric oxide (NO) is a vasodilator which inhibits platelet activation and has antiproliferative effects in vascular smooth muscle. Its effects are mediated through cGMP; cGMP is degraded by phosphodiesterase type-5 (PDE-5). These drugs inhibit PDE-5 and increase the levels of nitric oxide. The pulmonary vasculature contains large amounts of PDE-5. Two PDE-5 inhibitors have been investigated in RCTs in PAH (table 2).
Sildenafil
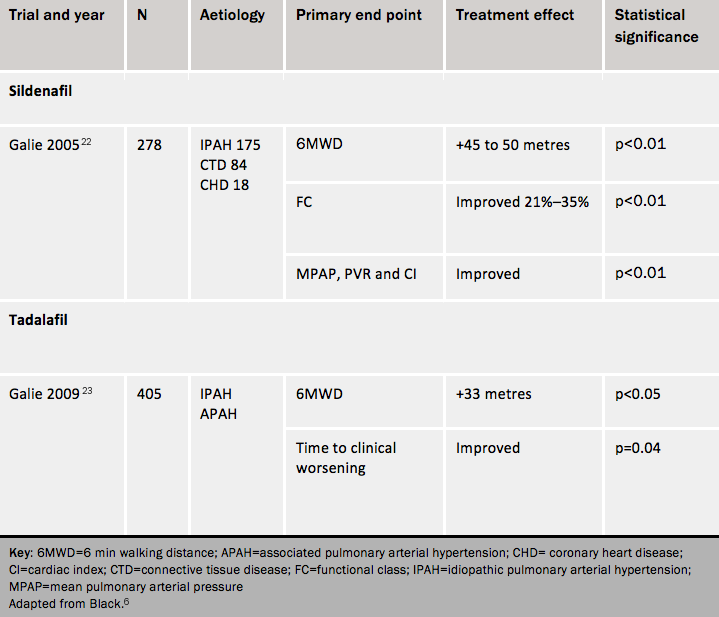
A randomised controlled trial of sildenafil produced favourable results with respect to exercise capacity, symptoms, functional class and haemodynamics.22 At 12 weeks, the 6MWT improvement was not statistically different between patients treated with sildenafil 20 mg three times daily and patients treated at higher doses. Long-term data in patients completing one year of sildenafil monotherapy at 80 mg tds showed sustained improvement in exercise capacity. Sildenafil is licensed for use at 20 mg tds only.
Side effects of sildenafil include headache, flushing, epistaxis, indigestion, diarrhoea and myalgia. It is contra-indicated in patients who have previously had gastrointestinal haemorrhage. Sildenafil is contraindicated in any patient taking nitrates.
Tadalafil
Tadalafil is taken once a day. It has a favourable effect on symptoms, exercise capacity, haemodynamics and time to clinical worsening at 40mg once daily at 12 weeks.23
Side effects (see SPC) include headache, dyspepsia, flushing, and nasal congestion. It is contra-indicated in certain groups of patients with cardiovascular disease, in those who are using any form of organic nitrate, and in patients with non-arteritic anterior ischaemic optic neuropathy.
Learning points
- Endothelial dysfunction occurs in PH, with reduced availability of NO (and hence a tendency to vasoconstriction)
- Drugs which augment NO availability, such as PDE-5 inhibitors, can improve symptoms
Endothelin receptor antagonists
Activation of the endothelin system has been demonstrated in patients with pulmonary arterial hypertension, though it is not certain whether raised plasma endothelin-1 levels are a cause or consequence of pulmonary hypertension. Endothelin-A and endothelin-B receptors are present in smooth muscle cells of the pulmonary vessels: through them, endothelin-1 exerts vasoconstrictor and mitogenic effects. Endothelin-B receptors are also present in endothelial cells: here, activation leads to release of vasodilators and antiproliferative molecules such as nitric oxide and prostacyclin. Data from clinical trials show no obvious clinical difference between endothelin A blockers and combined endothelin A and B receptor blockers (table 3).
Two endothelin receptor antagonists (ERAs) are in current clinical use — ambrisentan and bosentan. Sitaxsentan was withdrawn in 2010 for reasons of safety. They ERAs have the potential for liver injury, teratogenicity and falling haemoglobin. Patients require monthly monitoring of liver function, a monthly pregnancy test in women of child-bearing potential and three-monthly monitoring of haemoglobin levels. The main effect on liver function is to cause a transaminitis, which is reversible in the vast majority of patients by reducing the dose or discontinuing the drug according to the manufacturer’s instructions. Other side effects include peripheral oedema and nasal congestion.
Ambrisentan
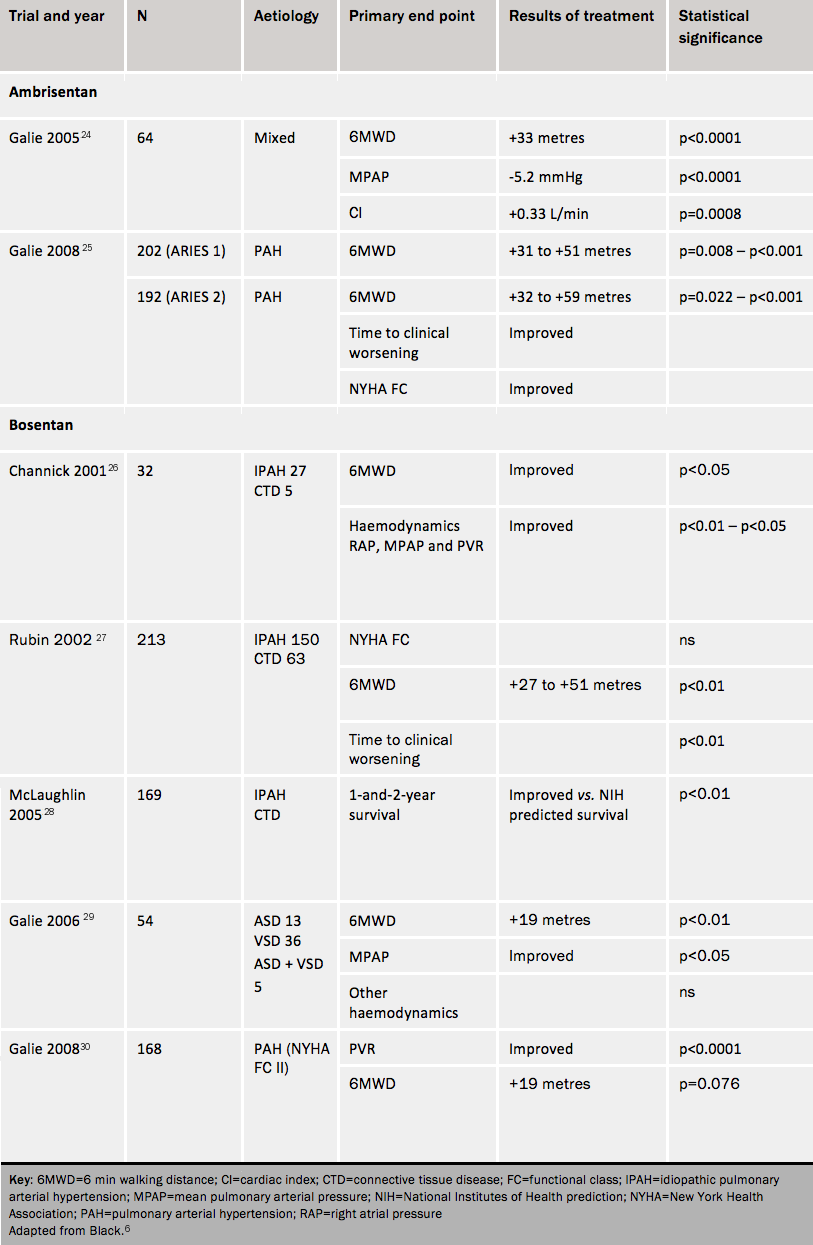
Ambrisentan improves symptoms, exercise capacity, haemodynamics and time to clinical worsening. These effects continue out to at least one year. It is given once a day at a dose of 5 mg, which can be increased to 10 mg if the 5 mg dose does not cause adequate improvement.
Bosentan
Bosentan was the first oral agent licensed for use in PAH. Bosentan improves exercise capacity, cardiac index, functional class and other haemodynamic indices, and reduces time to clinical worsening.
Long-term survival data28 indicate that first-line therapy with bosentan, with the addition of other therapy or change to other therapy as needed, resulted in survival estimates of 96% at one year and 89% at two years. Some 85% and 70%, respectively, were alive and on bosentan therapy alone at the end of one and two years.
Compared with placebo, in patients with Eisenmenger syndrome bosentan reduced pulmonary vascular resistance and mPAP and increased exercise capacity without worsening oxygen saturation.
A trial of patients in WHO functional class II improved pulmonary vascular resistance and time to clinical worsening.
Bosentan also appears to give improvements in patients with PAH associated with HIV infection.
Learning points
- Endothelin is a potent vasoconstrictor
- Endothelin receptor antagonists are drugs that block the action of endothelin and improve pulmonary vascular remodelling
Combination therapy in pulmonary hypertension
Combination therapy is standard practice for other forms of cardiovascular disease such as heart failure and hypertension. Pulmonary hypertension is another disease that involves multiple molecular pathways: the option of targeting multiple pathways in this condition in order to maximise efficacy is appealing, especially since many patients have a suboptimal response or develop tolerance to an initial therapeutic regimen.6
Drug therapy is administered with the intention of patients reaching clinical efficacy targets created for individual patients. Failure to achieve these targets after the peak effect of the drug should have been reached triggers clinical reassessment with a view to combination therapy. The suggested approach to clinical reassessment is presented in table (ESC table). Current practice is to use this sequential approach except in patients in WHO FC IV, in whom oral combination therapy is used when intravenous epoprostenol is not suitable. The value of such “upfront” combination therapy is the subject of at least one current RCT. The approach of waiting until the patient deteriorates to add a second drug is inappropriate because clinical deterioration in a patient with PAH is difficult to recover. Clinicians should refer to the SPC of the drugs used in pulmonary hypertension.
Table 4 summarises the results of combination drug trials in PAH. Although some of the earlier trials yielded disappointing results, more recent studies have demonstrated benefit. Possibly the largest challenge is the choice of primary end point in combination therapy trials. The six-minute walk test, which was the predominant primary end point in monotherapy placebo-controlled RCTs, has shown less improvement in combination therapy trials. This is partly a consequence of a ceiling effect of this test: the further the distance walked at baseline, the less improvement can be achieved on treatment.
Patients entering clinical trials in the current era tend to have milder symptoms and walk further at baseline. This is leading to the use of primary end points such as time to clinical worsening and its variants, which are favoured by regulatory authorities because this is a clinical rather than a surrogate measurement. Increased duration of trials beyond the typical 12-16 weeks of monotherapy trials as well as changes in trial design are likely to yield more useful data about clinical outcomes. Time to clinical worsening is an end point which combines mortality, hospitalization for PAH and various measures of clinical deterioration usually including WHO functional class and 6MWT distance.
Combination therapy results with prostanoids
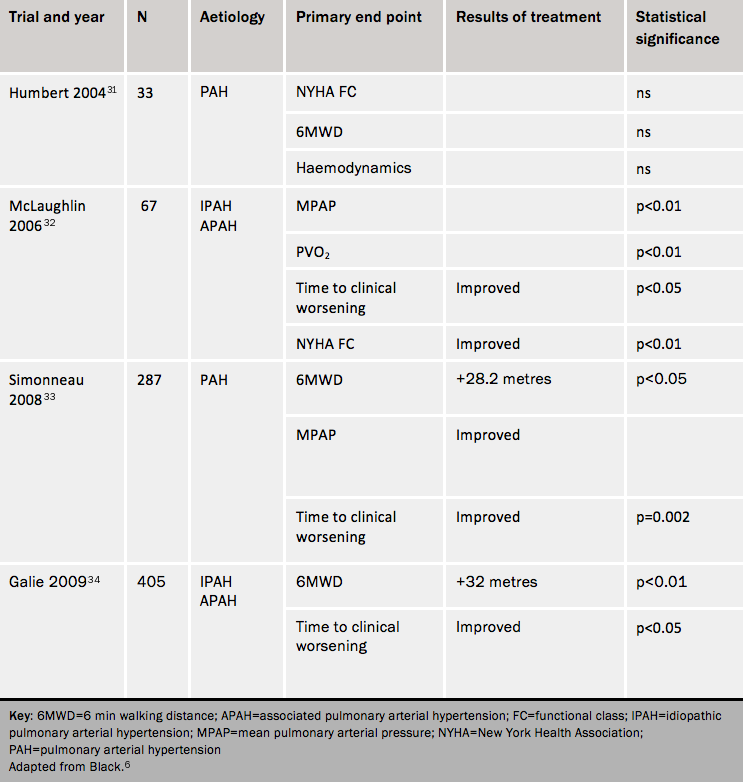
The BREATHE-2 (Bosentan Randomised trial of Endothelin Antagonist THErapy) study31 studied class III and IV patients starting on epoprostenol and randomised to bosentan or placebo. It showed a trend towards better haemodynamics with the combination.
The STEP-1 (Safety and pilot efficacy Trial of inhaled Iloprost in combination with bosentan for Evaluation in Pulmonary arterial hypertension) trial32 studied the effects of 12 weeks on inhaled iloprost in addition to bosentan. Combination therapy improved time to clinical worsening and functional class, and gave a marginal increase in 6MWT distance. However, the COMBI (COMbination therapy of Bosentan and aerosolized Iloprost in idiopathic pulmonary arterial hypertension) trial, of similar design was stopped early when a planned futility analysis showed no effect of combination treatment.
The TRIUMPH study of inhaled treprostinil in patients already taking bosentan or sildenafil reported a change in 6MWT of 15-20 metres compared with placebo but no change in functional class or time to clinical worsening. The PACES (Pulmonary Arterial hypertension Combination study of Epoprostenol and Sildenafil) trial showed improvements in 6MWT, haemodynamic indices and time to clinical worsening at 16 weeks when sildenafil was added to epoprostenol.33
Combination therapy results with ERAs and PDE-5 inhibitors
There is a pharmacokinetic interaction between bosentan and sildenafil: co-administration leads to a fall in plasma sildenafil levels and a rise in plasma bosentan levels. This has uncertain implications for efficacy and safety, but in the EARLY (Endothelin Antagonist tRial in mildly symptomatic pulmonary arterial hypertension patients) trial the haemodynamic effects were similar in patients given bosentan who were already taking sildenafil and in those taking bosentan alone.
In the PHIRST (Pulmonary arterial Hypertension and ReSponse to Tadalafil) study,34 tadalafil and bosentan given together gave a slight improvement in exercise capacity.
In conclusion, many questions remain concerning the choice and combination of drugs that may be used safely and effectively in pulmonary hypertension. Patients should be treated within trials or registries wherever possible.
Learning points
- PH patients may increasingly receive drug combinations in the future
- Vigilance is required to avoid adverse drug interactions
