Lowering serum cholesterol with statins has consistently shown benefits on cardiovascular outcomes. A 1 mmol/L reduction in low-density lipoprotein (LDL)-cholesterol is associated with approximately one-third fewer coronary events and one-fifth fewer ischaemic strokes.1 However, despite these impressive results, there remains a substantial residual risk of cardiovascular (CV) events despite optimal statin therapy.2 From pooled analyses of randomised-controlled trials of statins, there is a clear relationship between the achieved level of LDL-cholesterol and the number of coronary heart disease (CHD) events. This observation applies to both primary and secondary prevention trials.3
Concern has been expressed that very low levels of cholesterol might be associated with untoward adverse events, but there are two arguments against this hypothetical scenario. First, there are several remote societies, unexposed to ‘western’ diets, high in saturated fat, where very low levels of cholesterol are the norm,4 and there is no evidence that they suffer as a consequence. Second, data from the cholesterol-lowering trials show that proportional risk reductions in CV events are independent of baseline levels of cholesterol and those with the lowest levels of cholesterol gain the same relative risk reductions in CV outcomes.5 Additionally, those patients who achieve low levels of cholesterol in intervention trials do not experience an excess of adverse events potentially related to statin treatment.
The challenge
Today’s challenge is, therefore, to establish whether it is possible to improve CV outcomes by additional therapies which enable lower levels of cholesterol to be attained, particularly in patients at higher CV risk, including those with established CV disease, and those with hypercholesterolaemia, in whom optimal levels of cholesterol cannot be achieved with existing statin therapies. It is worth emphasising that while more patients in the UK achieve therapeutic control of LDL-cholesterol than most, if not all, other European countries, more than one third of patients in the UK do not.6 There is, therefore, substantial residual CV risk in these patients, and novel therapies that provide additional cholesterol lowering offer exciting opportunities to improve CV outcomes.
In addition, while in randomised, placebo-controlled trials, statins are extremely well tolerated (and adverse events are recorded almost as frequently in the placebo group as in those assigned a statin!), in observational studies and clinical practice, side effects, particularly myalgia, attributed to statin use, are much more commonly reported, and lead to discontinuation of statin therapy.7 Thus, there is a real need for alternative lipid-lowering therapies that are not only effective, but also well tolerated.
In an accompanying commentary by Gilbert Wagener, he raises doubts about the incremental benefits of achieving lower LDL-cholesterol goals, highlights the potentially high number needed to treat (NNT), particularly in those at low CV risk, and the cost-effectiveness of new treatments. However, he ignores the proportion of high CV risk patients (>67% in EUROASPIRE III6 – European Action on Secondary and Primary Prevention by Intervention to Reduce Events III), who for various reasons never achieve even conservative LDL-cholesterol targets with conventional statin-based treatment, and who remain at an unacceptable risk of CV events. While underuse of optimal statin therapy contributes to this high percentage, hypercholesterolaemia, statin intolerance and other factors demand the search for, and trial of, new therapies that are effective, safe and well tolerated. Cost-effectiveness analyses will be applied to these new studies and, ultimately, guideline developers and healthcare providers will dictate their place in clinical practice.
PCSK9 (proprotein convertase subtilisin kexin type 9) and inhibitors of PCSK9
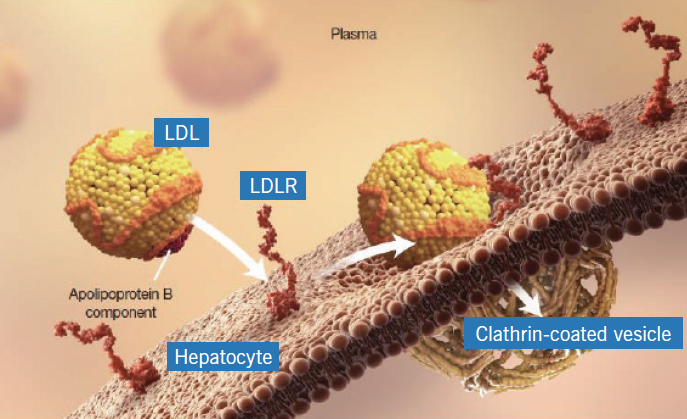
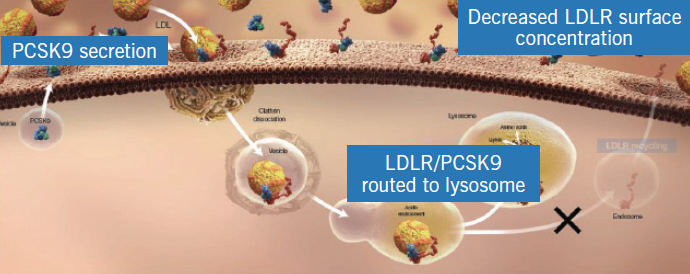
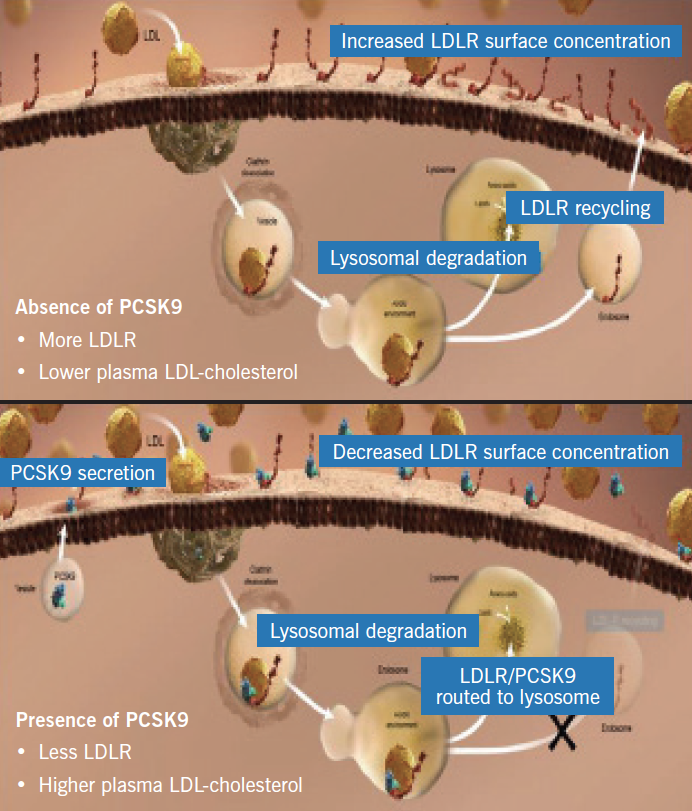
The LDL-receptor on the surface of hepatocytes plays a central role in cholesterol homeostasis. Circulating LDL-cholesterol binds to the LDL-receptor and the complex is internalised in the hepatocyte in clathrin-coated vesicles, the contents of which undergo lysosomal degradation (figure 1). The LDL-receptor is, however, recycled to the cell surface where it can bind with more LDL-cholesterol molecules (figure 2). PCSK9 is a protein synthesised by the hepatocytes, which binds to the LDL-receptor component of the LDL-receptor/LDL-cholesterol complex. However, when this complex is internalised in the hepatocyte, the resultant lysosomal degradation does not permit the LDL-receptor to be recycled to the cell membrane (figure 3). Thus, the number of LDL-receptors on the surface of the hepatocytes is reduced and serum levels of LDL-cholesterol increase. The more PCSK9 produced by the liver, the higher the LDL-cholesterol level.
Genetic variations in PCSK9 are associated with high or low levels of the protein, which, in turn, relate to high and low levels of LDL-cholesterol (figure 4) and, not surprisingly, high and low risks of CHD, respectively. PCSK9 is, therefore, a novel regulator of hepatic LDL-receptor expression and an obvious target to influence levels of LDL-cholesterol.
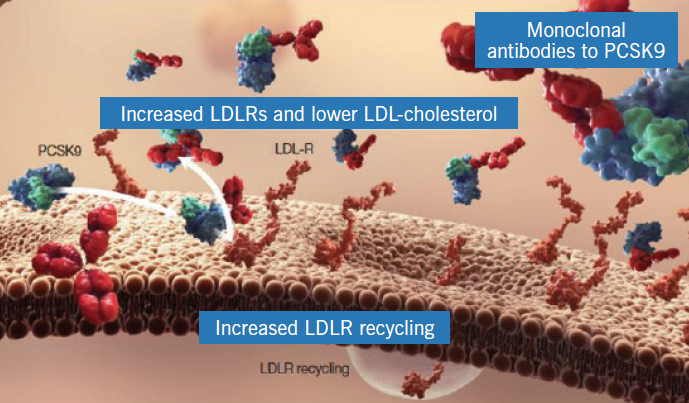
Monoclonal antibodies have been developed that bind PCSK9 and prevent its interaction with the LDL-receptor (figure 5), thereby preserving receptor numbers and lowering LDL-cholesterol. In phase II clinical trials the administration of human monoclonal antibodies to PCSK9 has been associated with impressive reductions of LDL-cholesterol (50–70%), not only in patients who were statin naïve, but also in patients receiving optimal doses of high-intensity statins, including those with heterozygous hypercholesterolaemia.8,9 In these studies, highly significant reductions were also seen in apolipoprotein B (ApoB), lipoprotein(a) and triglycerides.
Overall, the monoclonal antibodies tested thus far have been shown to be not only effective, but safe and well tolerated. Because of their nature they have to be administered by subcutaneous injection either two weekly or four weekly. Novel auto-injector devices make subcutaneous administration of the antibody relatively simple and cause minimal discomfort.
Long-term efficacy and safety trials are now the major challenge, and two large phase III morbidity/mortality outcome trials are currently ongoing, designed to establish whether, in patients with established CV disease (CVD), the administration of a monoclonal antibody to PCSK9 confers additional protection against CV events on top of optimal statin therapy.
FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk; sponsored by Amgen Inc.) is a trial of 22,500 patients with established CVD, recruited on the basis of a past history of myocardial infarction, stroke or symptomatic peripheral vascular disease. Patients enter the study on optimal background statin therapy (usually atorvastatin 40–80 mg), with an LDL-cholesterol ≥1.8 mmol/L or non-high-density lipoprotein (non-HDL)-cholesterol ≥2.6 mmol/L. They are randomised to the monoclonal antibody, evolocumab, or placebo administered by subcutaneous injection two or four weekly. Patients will be followed up for an average of about four years until approximately 1,630 hard end points have occurred (non-fatal myocardial infarction, non-fatal stroke and fatal CVD). The trial is designed to detect a 15% reduction in this combined end point in the active treatment group compared with placebo.
ODYSSEY OUTCOMES (Evaluation of Cardiovascular Outcomes after an Acute Coronary Syndrome During Treatment With Alirocumab; sponsored by Sanofi and Regeneron) is a trial of 18,000 patients recruited within one year of an acute coronary syndrome (ACS) and who, despite high-intensity or maximum tolerated dose of statin, have an LDL-cholesterol >1.8 mmol/L. Patients are randomised to alirocumab or placebo as a two weekly subcutaneous injection. The principal outcome is the composite of CHD death, non-fatal myocardial infarction, ischaemic stroke or unstable angina. The expected duration of the study is four years, by which time approximately 1,650 primary events are predicted to have occurred. With a placebo event rate of 11.4% at four years, the study is powered to test a 15% reduction in the primary end point.
Optimal population
Clearly, both these trials are carried out in high-risk patients with established CVD, who are already receiving optimal or best-tolerated statin treatment. The main difference is in the patient population. The focus of ODYSSEY OUTCOMES is limited to subjects with recent ACS, while FOURIER includes subjects who have suffered a myocardial infarction or ischaemic stroke at any time, and patients with peripheral vascular disease. The benefits of lipid-lowering with statins have been established in all three of these groups, however, despite guidelines advocating their widespread use, in everyday clinical practice, these patients are suboptimally treated. Currently, in the UK, only about 50% of ischaemic stroke patients are receiving effective long-term statin therapy.
Further information on both these trials is available on clinicaltrials.gov. Additional information on the FOURIER study, including details of all UK participating sites, can be found at: http://www1.imperial.ac.uk/clinicaltrialsunit/currenttrials/
Acknowledgement
Figures 1–5 are reproduced with permission from Amgen Inc.
Conflict of interest
Peter Sever is a member of the Executive Committee for the FOURIER trial.
Editors’ note
See also the editorial by Gilbert Wagener in this issue.
References
1. Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–78. http://dx.doi.org/10.1016/S0140-6736(05)67394-1
2. Kastelein JJP. The realities of dyslipidaemia: what do the studies tell us? Europ Heart J Suppl 2005;7:F27–F33. http://dx.doi.org/10.1093/eurheartj/sui040
3. Kastelein JJP. The future of best practice. Atherosclerosis 1999;143(suppl 1):S17–S21. http://dx.doi.org/10.1016/S0021-9150(99)00103-3
4. Hochholzer W, Giugliano RP. Lipid lowering goals: back to nature? Ther Adv Cardiovasc Dis 2010;4:185–91. http://dx.doi.org/ 10.1177/1753944710368206
5. Cholesterol Treatment Trialists’ (CTT) Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 2012;380:581–90. http://dx.doi.org/10.1016/S0140-6736(12)60367-5
6. Reiner Z, De Bacquer D, Kotseva K, Prugger C, De Backer G, Wood D; EUROASPIRE III Study Group. Treatment potential for dyslipidaemia management in patients with coronary heart disease across Europe: findings from the EUROASPIRE III survey. Atherosclerosis 2013;231:300–07. http://dx.doi.org/10.1016/j.atherosclerosis.2013.09.020
7. Abd TT, Jacobson TA. Statin-induced myopathy: a review and update. Expert Opin Drug Saf 2011;10:373–87. http://dx.doi.org/10.1517/14740338.2011.540568
8. Stein EA, Swergold GD. Potential of proprotein convertase subtilisin/kexin type 9 based therapeutics. Curr Atherosclerosis Reports 2013;15:310. http://dx.doi.org/10.1007/s11883-013-0310-3
9. Koren MJ, Giugliano RP, Raal FJ et al.; OSLER investigators. Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER) randomised trial. Circulation 2014;129:234–43. http://dx.doi.org/10.1161/CIRCULATIONAHA.113.007012

