Hola Madrid! More than 1,800 clinicians and scientists from 75 countries converged on Madrid, Spain, for the 52nd annual congress of the European Atherosclerosis Society (EAS), held 31 May–3 June at the Institución Ferial de Madrid. At the opening ceremony EAS President, Professor Alberico Catapano (University of Milan and Multimedica IRCCS, Italy) emphasised that 2014 marks the start of an exciting time in lipid research, with ongoing studies evaluating different approaches to managing patients at high cardiovascular risk, as well as exciting new discoveries in basic research leading to a better understanding of atherosclerotic diseases.
Lowering LDL-cholesterol: we need to do better
 It is essential that high-risk patients attain the recommended low-density lipoprotein (LDL)-cholesterol target. As reported at the first late-breaking session, the choice and dose of statin are key factors influencing LDL-cholesterol lowering. In a meta-analysis of the VOYAGER (Individual Patient Data Meta-analysis of Statin Therapy in At-risk Groups: Effects of Rosuvastatin, Atorvastatin and Simvastatin) database of 37 studies of high-intensity statins, 71% of patients treated with rosuvastatin 40 mg achieved at least 50% reduction in LDL-cholesterol levels, compared with 59% for atorvastatin 80 mg, and 57% versus 40% for rosuvastatin 20 mg and atorvastatin 40 mg, respectively.1
It is essential that high-risk patients attain the recommended low-density lipoprotein (LDL)-cholesterol target. As reported at the first late-breaking session, the choice and dose of statin are key factors influencing LDL-cholesterol lowering. In a meta-analysis of the VOYAGER (Individual Patient Data Meta-analysis of Statin Therapy in At-risk Groups: Effects of Rosuvastatin, Atorvastatin and Simvastatin) database of 37 studies of high-intensity statins, 71% of patients treated with rosuvastatin 40 mg achieved at least 50% reduction in LDL-cholesterol levels, compared with 59% for atorvastatin 80 mg, and 57% versus 40% for rosuvastatin 20 mg and atorvastatin 40 mg, respectively.1
Yet according to DYSIS (Dyslipidemia International Study) in more than 21,000 patients at very high cardiovascular risk, while the use of statins has increased in Europe, most were prescribed at lower doses, equivalent to 20–40 mg simvastatin. Higher doses were used by only 15% and the combination of statin plus ezetimibe by even fewer (9%). As a result, less than one in five patients achieved their LDL-cholesterol target.2
These findings are highly relevant to the UK National Institute for Health and Care Excellence (NICE) guidelines – then in draft guidance, but now published – which recommend wider use of statins for lowering LDL-cholesterol, with the threshold for starting preventive treatment halved from a 20% risk of developing cardiovascular disease (CVD) over 10 years to a 10% risk.3 This has been hotly debated by experts arguing against over-medicalisation of lower-risk patients.4 Most recently, the British Cardiovascular Society has weighed in to reaffirm the importance of treating high-risk patients (the full press release can be found here).5
How best to manage residual cardiovascular risk?
Yet even if high-risk patients achieve LDL-cholesterol targets, they remain at high residual risk of cardiovascular events.

The evidence for therapeutic approaches beyond statins was the focus of one plenary lecture, given by Professor Jane Armitage (University of Oxford, UK). Fibrate therapy may be considered in statin-treated patients with residual hypertriglyceridaemia or mixed dyslipidaemia, largely based on subgroup analyses from the ACCORD (Action to Control Cardiovascular Risk in Diabetes) Lipid trial (see our podcast from the meeting).6 Combination treatment with ezetimibe plus statin may improve LDL goal attainment, although the jury is still out on potential outcomes benefit, pending the results of IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial), expected later this year.
This is covered in more detail in our podcast from the EAS. Novel approaches to lipid lowering clearly offer potential. Professor Armitage highlighted two strategies, cholesteryl ester transfer protein (CETP) inhibitors and inhibitors of proprotein convertase subtilisin kexin 9 (PCSK9) (see our podcast from the meeting).
CETP inhibitors have had a rocky ride in development, although those currently in development have been shown to provide efficacious lipid modification with no adverse signal to date. In another plenary session, Professor Anne Tybjaerg-Hansen (University Hospital, Copenhagen, Denmark) highlighted a Mendelian randomisation study (a type of ‘natural’ randomised study) in which individuals with genetic variants for CETP, resulting in lifelong exposure to raised high-density lipoprotein (HDL)-cholesterol and decreases in triglycerides, LDL-cholesterol, and non-HDL cholesterol, had a reduced risk of CVD.7
These findings suggest promise for the CETP inhibitors, although definitive proof is awaited from the results of the REVEAL (Randomized Evaluation of the Effects of Anacetrapib Through Lipid- Modification) trial with anacetrapib, co-ordinated by the Oxford Clinical Trials Unit, and the ACCELERATE (Aliskiren and the Calcium-Channel Blocker Amlodipine Combination as an Initial Treatment Strategy for Hypertension) trial with evacetrapib, expected in 2016/2017.
The second strategy, PCSK9 inhibition, was a major focus of the congress. Professor Armitage talks about these two novel approaches in the BJC podcast (click here to view).
Congress buzzword: PCSK9
PCSK9 was a key focus of discussion at EAS Madrid. As highlighted by Dr Bertrand Cariou (INSERM UMR 1087, Nantes, France) in a plenary lecture, PCSK9-targeted therapies represent a genomic revolution in drug development, with findings from genetic studies translating to novel therapies in less than 10 years. The most advanced are monoclonal antibody therapies targeting PCSK9 which, given evidence of substantial LDL-cholesterol lowering, offer potential for reducing residual cardiovascular risk in three key areas of unmet patient needs: high-risk patients with CVD, statin intolerance and familial hypercholesterolaemia (FH).
FH is more prevalent than previously thought. Professor JJP Kastelein (Academic Medical Center, University of Amsterdam, the Netherlands) reviewed recent studies which show that heterozygous FH affects between one in 200 and one in 250 people in Europe.8,9 The burden of markedly elevated LDL-cholesterol from birth causes premature coronary heart disease if untreated; however, if patients with heterozygous FH are diagnosed early and treated, they can live a normal life. The EAS has recently highlighted the extent of underdiagnosis and undertreatment of FH in a position statement (available in full here), widely cited internationally.9 A consensus statement from the EAS on homozygous hypercholesterolaemia is also in press.
The International FH Foundation has also recently published guidance on FH management (available in full here) including a focus on best practice in cascade screening for FH, generally consistent with NICE guidance.10

Lead author of the IFH Foundation guidelines, Professor Gerald Watts (University of Western Australia, Perth, Australia) commented: “These guidelines are aimed at closing the gap in the care of FH, including the integration of care between primary care physicians and lipid specialists.” He talks to the BJC in our podcast from the EAS.
Diet and statins are the mainstay of treatment in FH. Further support for the use of statins in children and adolescents (aged 6–17 years) with FH is provided by the CHARON (Hypercholesterolaemia in Children and Adolescents Taking Rosuvastatin Open Label) study, in which treatment with rosuvastatin (5–20 mg/day) reduced LDL-cholesterol substantially (by 35-45% at two years) and was well tolerated.11 Rosuvastatin did not significantly impact atherosclerosis (assessed by carotid intima-media thickness), although there was a trend for slowing of progression versus untreated siblings.12 Additionally, prolonged use of statins in FH was not associated with increased risk of new-onset diabetes.
A report from the Academic Medical Center (University of Amsterdam, the Netherlands) showed that the risk for new-onset diabetes was 50% lower in FH patients with LDL receptor mutations compared with individuals without FH; in patients with more severe LDL receptor mutations, diabetes risk was even lower. This suggests that the LDL receptor is the common causal pathway underlying the statin-induced increase in diabetes risk and the FH-associated decrease in diabetes risk.13
However, there is a gap in FH management, as most patients do not achieve LDL-cholesterol targets with current treatment. PCSK9 monoclonal antibody therapy has already been shown to be effective in further lowering LDL-cholesterol on top of statins in patients with heterozygous FH, and was well tolerated.14,15 At EAS Madrid, a late-breaking clinical report16 also showed benefit in patients with homozygous FH, the most severe form of FH. The phase III TESLA (Trial Evaluating PCSK9 Antibody in Subjects with LDL Receptor Abnormalities) study randomised 50 patients (aged 13–57 years, 43% already with manifest coronary artery disease, baseline LDL-cholesterol 9.0 mmol/L on intensive statin ±ezetimibe) to the PCSK9 monoclonal antibody therapy evolocumab (420 mg subcutaneously, every month) or placebo. Compared with placebo, treatment with evolocumab reduced LDL-cholesterol by 31% overall. There was no significant adverse signal associated with this treatment.
PCSK9: outstanding questions
With the drive for personalised medicine, a key consideration is how best to customise PCSK9 treatment, taking into account patient needs, background lipid-lowering therapy and LDL-cholesterol target. This was discussed at one of the educational symposia.
PCSK9 levels vary considerably within the population. Statins are known to up-regulate PCSK9 levels, posing the question of whether a lower dose regimen of the PCSK9 monoclonal antibody would provide sufficient PCSK9 inhibition and LDL-cholesterol lowering in statin-intolerant patients not on treatment. Dr Michel Farnier (Point Medical, Dijon, France) discussed findings from ODYSSEY Mono (Efficacy and Safety of Alirocumab SAR236553 (REGN727) Versus Ezetimibe in Patients With Hypercholesterolemia), one of the studies in the ODYSSEY clinical trials programme with the PCSK9 monoclonal antibody therapy alirocumab. In patients not on lipid-lowering therapy, alirocumab 75 mg every two weeks produced effective LDL-cholesterol lowering (by 55% at 12 weeks), and in the majority of patients, this dose was sufficient to provide sustained LDL-cholesterol lowering and inhibition of PCSK9 levels over 24 weeks.17
Another question is whether non-statin therapy, such as ezetimibe or a fibrate, influences the response to PCSK9 monoclonal antibody therapy. Insights from a recent study suggest this is not the case, with similar LDL-cholesterol lowering with alirocumab 150 mg every four weeks when given as monotherapy or in combination with ezetimibe or fenofibrate.18
Together, these findings provide the rationale for a new study with alirocumab, ODYSSEY Choice II in patients with primary hypercholesterolaemia not treated with a statin. This study is comparing alirocumab 75 mg every two weeks or 150 mg every four weeks, with the possibility of up-titration to allow for a tailored therapeutic approach.19
Diet at the heart
Finally, there was renewed focus on lifestyle intervention as the first fundamental step in CVD prevention, particularly relevant for patients at low-to-medium cardiovascular risk. Professor Luis Masana (University Hospital Sant Joan, Spain) commented that small changes in lifestyle in the lower-risk patient can translate to important clinical benefits over the longer-term. Such is the thinking behind the lifestyle approach to cardiovascular risk assessment adopted in the recent Joint British Societies guidelines (JBS3) (for more information, click here).
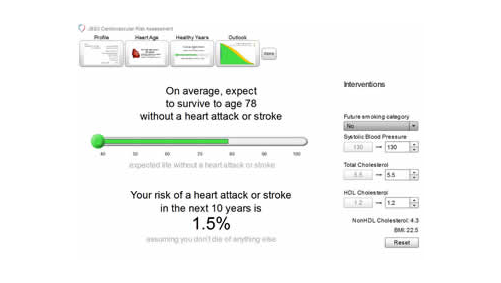
However, the key issue with lifestyle is sustaining changes in the long-term. In response, the EAS have advised on the development of an e-learning tool – the Dyslipidaemia Management Tool – which provides important information on CVD and associated risk factors, tips on diet and lifestyle, functional foods and approaches to assist in motivating and sustaining lifestyle change. This tool is expected to launch during the summer of 2014.
References
1. EAS-1166. Karlson BW, Palmer MK, Nicholls SJ, Lundman P, Barter PJ. Attainment of the anticipated ³50% reduction in LDL-C in the four ACC/AHA guidelines statin benefit groups: a VOYAGER meta-analysis. Late-breaking oral presentation, EAS Madrid 2014.
2. EAS-0421. Lautsch D, Gitt AK, Brudi P, Vanneste B, Ambegaonkar B. Statin-treated patients at very high cardiovascular risk in Europe: are the majority close to LDL-C goal? Moderated poster presentation, EAS Madrid 2014.
3. National Institute for Health and Care Excellence. Lipid Modification (update): guideline consultation. Available at: http://www.nice.org.uk/guidance/index.jsp?action=folder&o=66546
4. Open letter to NICE, dated 10 June 2014. Available at: http://www.nice.org.uk/media/877/AC/NICE_statin_letter.pdf
5. British Cardiovascular Society press release. Response to open letter concerning NICE draft guidance on statins. Available at http://www.bcs.com/pages/news_full.asp?NewsID=19792253
6. Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–74. http://dx.doi.org/10.1056/NEJMoa1001282
7. Johannsen TH, Frikke-Schmidt R, Schou J, et al. Genetic inhibition of CETP, ischemic vascular disease and mortality, and possible adverse effects. J Am Coll Cardiol 2012;60:2041–8. http://dx.doi.org/10.1016/j.jacc.2012.07.045
8. Sjouke B, Kusters DM, Kindt I, et al. Homozygous autosomal dominant hypercholesterolaemia in the Netherlands: prevalence, genotype-phenotype relationship, and clinical outcome. Eur Heart J 2014. (Epub ahead of print). http://dx.doi.org/10.1093/eurheartj/ehu058
9. Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J 2013;34:3478–90a. http://dx.doi.org/10.1093/eurheartj/eht273
10. Watts GF, Gidding S, Wierzbicki AS, et al. Integrated guidance on the care of familial hypercholesterolaemia from the International FH Foundation. Int J Cardiol 2014;171:309–25. http://dx.doi.org/10.1016/j.ijcard.2013.11.025
11. EAS-0385. Braamskamp M, Langslet G, McCrindle BW, et al. Efficacy and safety of rosuvastatin in children aged 6–17 years with familial hypercholesterolemia: findings from the Charon Study. Moderated poster presentation, EAS Madrid 2014.
12. EAS-0604. Braamskamp MJAM, Langslet G, McCrindle BW, et al. Effect of rosuvastain therapy on carotid intima media thickness in children with familial hypercholesterolemia: findings from the Charon study. Oral presentation, EAS Madrid 2014.
13. EAS-0216. Besseling J, Hutten BA, Kastelein JJP, Hovingh GK. Lack of LDL-receptors protects against new-onset type 2 diabetes – results of a cohort of 38,000 individuals analysed for FH mutations. Oral presentation, EAS Madrid 2014.
14. Raal F, Scott R, Somaratne R, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation 2012;126:2408–17. http://dx.doi.org/10.1161/CIRCULATIONAHA.112.144055
15. Stein EA, Gipe D, Bergeron J, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet 2012;380:29–36. http://dx.doi.org/10.1016/S0140-6736(12)60771-5
16. EAS-1177. Raal F, Honarpour N, Blom DJ, et al. Trial evaluating evolocumab, a PCSK9 antibody in patients with homozygous FH (TESLA): results of the randomised, double-blind placebo-controlled trial. Late breaking oral presentation, EAS Madrid 2014.
17. EAS-0758. Farnier M, Kastelein JJP, Roth E, et al. Relationship between alirocumab, PCSK9 and LDL-C levels: results from the ODYSSEY MONO Phase 3 trial of alirocumab 75 mg every 2 weeks. Moderated poster presentation, EAS Madrid 2014.
18. Rey J, Poitiers F, Paehler T, et al. Randomized, partial blind study of the pharmacodynamics, pharmacokinetics and safety of multiple subcutaneous doses of alirocumab, a fully human monoclonal antibody to proprotein convertase subtilisin/kexin type 9, administered every 4 weeks alone or in combination with ezetimibe or fenofibrate in healthy subjects. Poster presentation at the American College of Cardiology Scientific Sessions 2014. Abstract 1183–131.
19. Phase III Study To Evaluate Alirocumab in Patients With Hypercholesterolemia Not Treated With a Statin (ODYSSEY CHOICE II). Available at https://clinicaltrials.gov/ct2/show/NCT02023879
