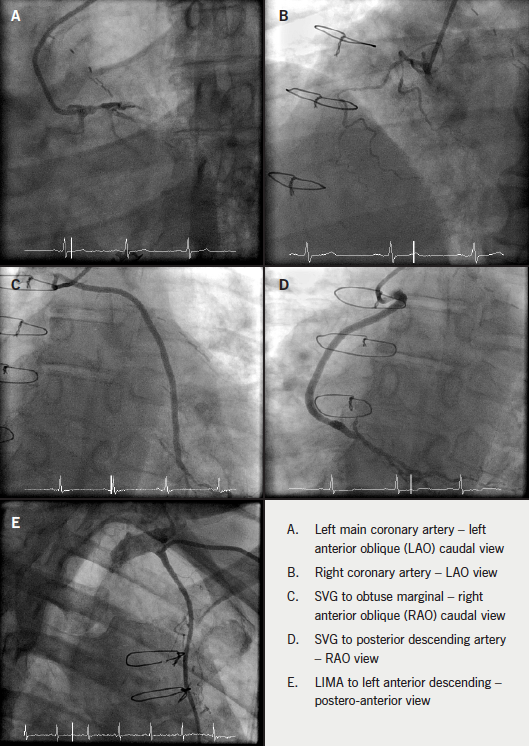
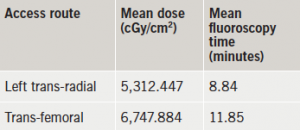
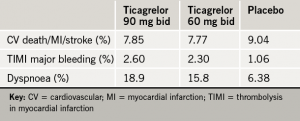
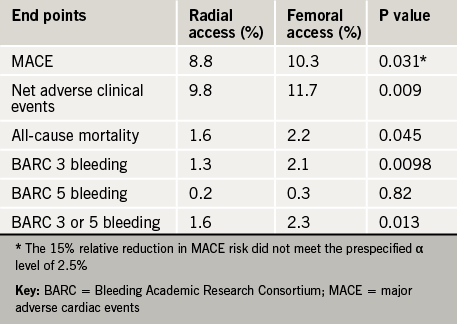
Glasgow was the host of the recent 83rd Annual Congress of the European Atherosclerosis Society (EAS), held from 22nd–25th March 2015 and attended by more than 1,500 delegates from across 77 countries. We report its highlights.
FH initiative
 Headlining the Congress was the launch of the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC), a consortium of major FH registries across Europe, Asia-Pacific, Africa and South America, led by Professor Kausik Ray (Imperial College, London). As shown by the previous EAS Consensus Panel statement, FH is one of the most common inherited conditions, yet it is underdiagnosed and undertreated in almost all countries.1 The FHSC will provide information on key aspects relating to FH care which will be critical in leveraging public policy to improve detection and management. Linking patient and clinician empowerment underpins the mission of the FHSC: in recognition of this common goal, EAS Glasgow brought together representatives of FH Patient Advocacy groups from around the world, including HEART UK − The Cholesterol Charity (http://heartuk.org.uk/), to gain insights into how barriers to optimum FH care can be overcome.
Headlining the Congress was the launch of the EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC), a consortium of major FH registries across Europe, Asia-Pacific, Africa and South America, led by Professor Kausik Ray (Imperial College, London). As shown by the previous EAS Consensus Panel statement, FH is one of the most common inherited conditions, yet it is underdiagnosed and undertreated in almost all countries.1 The FHSC will provide information on key aspects relating to FH care which will be critical in leveraging public policy to improve detection and management. Linking patient and clinician empowerment underpins the mission of the FHSC: in recognition of this common goal, EAS Glasgow brought together representatives of FH Patient Advocacy groups from around the world, including HEART UK − The Cholesterol Charity (http://heartuk.org.uk/), to gain insights into how barriers to optimum FH care can be overcome.
 With an estimated prevalence of one in 200–250 people, heterozygous FH accounts for most of the burden of FH care. Recent recognition of the genetic and phenotypic heterogeneity of homozygous FH2 has highlighted the need for further information into this severe FH presentation. This is the aim of a substudy of the EAS-FHSC Initiative − the HoADH International Clinical Collaboration (HICC) − jointly led by Professor Derick Raal (University of the Witwatersrand, Johannesburg, South Africa) and Dr G Kees Hovingh (Academic Medical Center, Amsterdam, the Netherlands). Professor Raal discusses why the FHSC, including homozygous autosomal dominant hypercholesterolaemia (HoADH), are critical for FH care worldwide in our podcast.
With an estimated prevalence of one in 200–250 people, heterozygous FH accounts for most of the burden of FH care. Recent recognition of the genetic and phenotypic heterogeneity of homozygous FH2 has highlighted the need for further information into this severe FH presentation. This is the aim of a substudy of the EAS-FHSC Initiative − the HoADH International Clinical Collaboration (HICC) − jointly led by Professor Derick Raal (University of the Witwatersrand, Johannesburg, South Africa) and Dr G Kees Hovingh (Academic Medical Center, Amsterdam, the Netherlands). Professor Raal discusses why the FHSC, including homozygous autosomal dominant hypercholesterolaemia (HoADH), are critical for FH care worldwide in our podcast.
Hot off the press
The Clinical Latebreaker session gave a tantalising insight into a prospective EAS Consensus Paper on paediatric FH expected shortly. In his presentation, Dr Albert Wiegman (Academic Medical Center, Amsterdam, the Netherlands) made the case for targeting children and adolescents with FH. Childhood provides the ideal opportunity for screening for FH on the basis of an elevated low-density lipoprotein cholesterol (LDL-C) value, given the lack of dietary and hormonal influences. Dr Wiegman presented data showing that if the child is not overweight and has normal thyroid function, an elevated LDL-C (>95th percentile) suggests that FH is highly likely. This EAS Consensus Paper will have important impact for all stakeholders in FH care.
Statins back in the news
Statins were again in the news at EAS Glasgow. Dr David Preiss (Institute of Cardiovascular and Medical Sciences, University of Glasgow) presented findings from a new collaborative meta-analysis of 17 statin trials including 132,568 subjects (with and without coronary disease), followed for an average of 4.3 years. Statin treatment led to a significant 10% reduction in first hospital admission for heart failure (relative risk 0.90, 95% CI 0.84 to 0.97). The study was published simultaneously in the European Heart Journal.3 Dr Preiss commented that with evidence of greater benefit from statins in the long-term, this analysis may underestimate the true benefit of statins. Already heart failure accounts for almost 2% of the UK NHS budget: with an ageing population, it has been estimated that this may increase by at least 50% over the next 25 years.4,5
There was also much interest in the recently published EAS Consensus Panel statement on statin associated muscle symptoms (SAMS),6 which featured in two key Educational Symposia. One session entitled ‘Statin Intolerance, an impactful and yet unresolved clinical challenge’ highlighted the clinical impact of SAMS, which is responsible for up to 40% of referrals to lipid clinics, according to Professor Erik Stroes (Academic Medical Center, Amsterdam, the Netherlands) co-chair of this session. Importantly, in most cases muscle pain is not associated with creatine kinase (CK) elevation. In support, a general practice study in Spain (n=3,845) showed that 78% of patients with muscle pain on statins did not have CK elevation.7 Strategies for identifying and managing SAMS were discussed; taking sufficient time with patients to discuss the need for statin treatment, investigating the symptoms with statin de-challenge and re-challenge, and considering a low dose of an alternative statin, were all key to management.
The other Educational Session ‘Latest developments in difficult-to-treat patients with hypercholesterolemia’ highlighted the limitations of current treatments for addressing the unmet needs of patients at high cardiovascular risk, including those with FH or SAMS. Novel agents, specifically PCSK9 monoclonal antibody therapy, clearly offer potential. Professor Stroes overviewed evidence from recent studies, including GAUSS-28 and ODYSSEY Alternative,9 in patients who were intolerant to at least two statins (including one at lowest dose in ODYSSEY Alternative). Both studies showed additional substantial LDL-C lowering with the PCSK9 inhibitor (either evolocumab or alirocumab, respectively), with no indication of increase in myalgia, CK elevation or muscle pain. Moreover, interim long-term safety data from ODYSSEY Alternative showed that only 0.7% of patients treated with alirocumab discontinued treatment due to muscle symptoms.9
Novel therapies: PCSK9 inhibitors
Much of the interest in recent meetings has been on PCSK9 inhibitors, and Glasgow was no exception. A new analysis of 4,166 patients in six phase III trials from the ODYSSEY programme, presented during the Clinical Latebreaker session, showed that the efficacy of PCSK9 inhibition was not blunted by background high-intensity statin therapy, or other lipid-lowering treatment.10 These findings provide important reassurance to clinicians, in the light of evidence that statins upregulate PCSK9 expression.11
A pooled analysis of 4,564 patients in eight phase 3 trials included in the ODYSSEY programme showed that treatment with the PCSK9 inhibitor alirocumab significantly improved LDL-C goal attainment; 75−79% on alirocumab attained LDL-C goal at week 24, compared with 52% on ezetimibe and 6−8% on statin alone.12 A key question, therefore, is what proportion of patients at high cardiovascular risk who are unable to attain LDL-C goal with current treatments would potentially qualify for PCSK9 inhibitor therapy? Evidence from DYSIS II (Dyslipidemia International Study II) provides some insight. Among 461 patients (76% male, mean age 64.1 years) with a recent acute coronary syndrome in Germany, only 22% attained the European Society of Cardiology/EAS guideline recommended LDL-C goal of 1.7 mmol/L (70 mg/dL).13 DYSIS II Belgium also showed that nearly half (46%) of patients with diabetes and coronary heart disease did not attain LDL-C goal.14 Furthermore, an analysis from LTAP-2 (the Lipid Treatment Assessment Project -2) including 9,955 patients (40% with atherosclerotic vascular disease) showed that 11.5% of patients were potential candidate for PCSK9 inhibitor treatment. However, if the LDL-C threshold was increased to >2.6 mmol/L (100 mg/dL), 21% of patients with cardiovascular disease would qualify.15
Reassertion of lifestyle
Lifestyle was also very much in the news at EAS Glasgow. In a special lecture ‘Investing in your arteries: importance of a healthy lifestyle for lifetime cardiovascular risk reduction’, Professor John Deanfield (British Heart Foundation Vandervell Professor of Cardiology, University College Hospital, Londonand lead author of the Joint British Societies Guidelines) argued for a renewed emphasis on ‘primordial prevention’, targeting cardiovascular risk factors such as smoking, cholesterol, blood pressure and body weight from an early age to optimise clinical benefit. He cited evidence from the Framingham Offspring Cohort which showed that long-term exposure (by 11−20 years) to even moderately elevated cholesterol was associated with about a four-fold higher risk of coronary events compared with individuals with no exposure before age 55 years.16 Professor Deanfield argued that public education should be a priority so that individuals can take responsibility for their cardiovascular health and sustain lifestyle change; this call for a primordial approach to cardiovascular disease prevention was echoed in a simultaneous publication in the European Heart Journal.17
In the EAS-International Chair on Cardiometabolic Risk (ICCR) Joint Session: ‘Changing lifestyle patterns: the challenge for cardiovascular prevention’, Professor Naveed Sattar (Institute of Cardiovascular and Medical Sciences, University of Glasgow) took a global view of the need for primordial prevention. Increasing urbanisation in developing economies has led to adoption of Westernised diets and increasing exposure to poor diet choices and physical inactivity, resulting in increasing obesity, diabetes and cardiovascular disease. The impact has been greatest in low to middle income countries: in Bangladesh, for example, diabetes prevalence in urban areas has increased seven-fold over the period 1986–2006. Smoking is a key challenge: in China, now with the world’s highest prevalence of smokers, one million people die each year due to smoking-related causes, and over the next 15 years this will triple.18 Professor Sattar emphasisesd the importance of focusing on lifestyle, and discusses the practicalities of sustaining change in our podcast.
A pertinent issue to this debate is the need for micronutrient supplementation, as discussed by Professor Ian Young (Centre for Public Health, Queen’s University Belfast, Northern Ireland). While experimental and epidemiological data, as well as studies in individuals with intermediate traits such as metabolic syndrome are supportive of benefit, clinical trials have less consistent results. Given that PREDIMED (Primary Prevention of Cardiovascular Disease with a Mediterranean Diet) 19 showed that inclusion of nuts or extra virgin olive oil in a Mediterranean diet reduced cardiovascular events (primarily stroke) by 30% in a high-risk primary prevention population, eating a diet rich in micronutrients rather than taking micronutrient supplements should be the preferred approach for preventing cardiovascular disease.
Novel biomarkers
The case for biomarkers for improving risk prediction especially in individuals at low to intermediate cardiovascular risk was a hot topic. Professor Kausik Ray emphasised the need to take account of the numbers needed to screen for treatment decisions and impact on clinical benefit.
Lipoprotein(a) (Lp(a)) is already established as a cardiovascular risk factor, independent of LDL-C or other lipids, mediated by pro-atherogenic, pro-thrombotic and antifibrinolytic effects which enhance atherothrombosis.20 Professor Børge Nordestgaard (University of Copenhagen & Copenhagen University Hospital, Denmark) discusses the evidence for Lp(a) and how to manage this in the clinic in our podcast.
New findings from an analysis of 98,097 subjects from the Copenhagen City Heart Study and the Copenhagen General Population Study link Lp(a) with risk for heart failure.21 Individuals with Lp(a) levels in the top 10% (68-289 mg/dL) had a 60−80% increase in heart failure (p<0.001 for trend). The effect of elevated Lp(a) was likely causal; the risk of heart failure was 18% (hazard ratio 1.18, 95% CI 1.04−1.34) for individuals carrying genetic variants known to be associated with elevated Lp(a), consistent with observational data (hazard ratio 1.22, 95% CI 1.11−1.35).
With the withdrawal of niacin/laropiprant in Europe, there are no therapeutic options that specifically target elevated Lp(a). Ongoing studies are evaluating the potential of an antisense oligonucleotide to apolipoprotein(a) contained on the Lp(a) molecule. Initial studies show 40−80% reduction in Lp(a) with doses of 100−300 mg; a phase 2 study in patients with elevated Lp(a) (>50 mg/dL) is ongoing.22
There was also news to strengthen the case for elevated non-fasting remnant cholesterol (defined as total cholesterol – HDL-C – LDL-C). This analysis included about 90,000 Danish individuals free of cardiovascular disease at baseline, of whom 4,435 developed ischaemic heart disease, 1,722 developed myocardial infarction, and 8,121 died over a 22-year follow-up period. Whereas both LDL-C and Lp(a) were associated equally with risk of ischaemic heart disease and myocardial infarction, only non-fasting remnant cholesterol concentrations were associated stepwise with increased risk of all-cause mortality. Subjects with the highest non-fasting remnant cholesterol levels (≥1.50 mmol/L) had a 60% increased risk of all-cause mortality.23
Accumulating evidence for these markers strengthens the case for consideration of their inclusion in future dyslipidaemia guidelines. Clearly, the lipid field continues to be ‘hot news’ for 2015 and beyond.
References
1. Nordestgaard BG, Chapman MJ, Humphries SE et al.; European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J 2013;34:3478−90a. http://dx.doi.org/10.1093/eurheartj/eht273
2. Cuchel M, Bruckert E, Ginsberg HN et al.; European Atherosclerosis Society Consensus Panel on Familial Hypercholesterolaemia. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J 2014;35:2146−57. http://dx.doi.org/10.1093/eurheartj/ehu274
3. Preiss D, Campbell RT, Murray HM et al. The effect of statin therapy on heart failure events: a collaborative meta-analysis of unpublished data from major randomized trials. Eur Heart J 2015 Mar 23. pii: ehv072. [Epub ahead of print]. http://dx.doi.org/10.1093/eurheartj/ehv072
4. National Institute for Health and Clinical Excellence. New NICE guidance will improve diagnosis and treatment of chronic heart failure. London: NICE, 2010. Link: http://www.nice.org.uk/newsroom/pressreleases/chronicheartfailureguidance.jsp
5. Sutherland K. Bridging the quality gap: heart failure. The Health Foundation, 2010. Link: http://www.health.org.uk/public/cms/75/76/313/583/Bridging%20the%20quality%20gap%20Heart%20Failure.pdf?realName=cXqFcz.pdf
6. Stroes ES, Thompson PD, Corsini A et al.; European Atherosclerosis Society Consensus Panel. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J 2015 Feb 18. pii: ehv043. [Epub ahead of print].
7. Pedro-Botet J, Millán Núñez-Cortés J, Flores JA, Rius J. Muscle symptoms related with statin therapy in general practice. 83rd Congress of the EAS, Glasgow, 22nd−25th March 2015. Abstract EAS-0232.
8. Stroes E, Colquhoun D, Sullivan D et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014;63:2541−8. http://dx.doi.org/10.1016/j.jacc.2014.03.019
9. Moriarty PM, Thompson PD, Cannon CP et al. ODYSSEY ALTERNATIVE: efficacy and safety of alirocumab versus ezetimibe, in patients with statin intolerance as defined by a placebo run-in and statin rechallenge arm. http://my.americanheart.org/idc/groups/ahamah-public/@wcm/@sop/@scon/documents/downloadable/ucm_469684.pdf
10. Krempf M, Bergeron J, Elassal J et al. Efficacy of alirocumab according to background statin intensity and other lipid-lowering therapy in heterozygous familial hypercholesterolemia or high CV risk populations: Phase 3 sub-group analyses. 83rd Congress of the EAS, Glasgow,22nd−25th March 2015. Abstract EAS-0493.
11. Mayne J, Dewpura T, Raymond A et al. Plasma PCSK9 levels are significantly modified by statins and fibrates in humans. Lipids Health Dis 2008;7:22. http://dx.doi.org/10.1186/1476-511X-7-22
12. Farnier M, Gaudet D, Valcheva V et al. Efficacy of alirocumab in heterozygous familial hypercholesterolemia or high CV risk populations: pooled analyses of eight phase 3 trials. 83rd Congress of the EAS, Glasgow, 22nd−25th March 2015. Abstract EAS-0563.
13. Gitt A, Ashton V, Horack M et al. Low LDL-C target achievement among treated ACS patients in Germany: The Dyslipidemia International Study (DYSIS) IIACS results. 83rd Congress of the EAS, Glasgow, 22nd−25th March 2015. Abstract EAS-0397.
14. Hermans M, Cools F, Gevaert S et al. Lipid abnormalities remain high among diabetic patients with stable CHD: results of the Dyslipidemia International Study (DYSIS) II Belgium. 83rd Congress of the EAS, Glasgow, 22nd−25th March 2015. Abstract EAS-0405.
15. Waters D, Santos R, Abreu P et al. Who might benefit from PCSK9 inhibitor treatment? An analysis from the Lipid Treatment Assessment Project-2 (LTAP-2). 83rd Congress of the EAS, Glasgow, 22nd−25th March 2015. Abstract EAS-0744.
16. Navar-Boggan AM, Peterson ED, D’Agostino RB Sr et al. Hyperlipidemia in early adulthood increases long-term risk of coronary heart disease. Circulation 2015;131: 451−8. http://dx.doi.org/10.1161/CIRCULATIONAHA.114.012477
17. Ayer J, Charakida M, Deanfield JE, Celermajer DS. Lifetime risk: childhood obesity and cardiovascular risk. Eur Heart J 2015 Mar 24. pii: ehv089. [Epub ahead of print]. http://dx.doi.org/10.1093/eurheartj/ehv089
18. World Health Organization. Smoking in China. http://www.wpro.who.int/china/mediacentre/factsheets/tobacco/en/
19. Estruch R, Ros E, Salas-Salvadó J et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279−90. http://dx.doi.org/10.1056/NEJMoa1200303
20. Nordestgaard BG, Chapman MJ, Ray K et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J 2010;31:2844−53. http://dx.doi.org/10.1093/eurheartj/ehq386
21. Nordestgaard BG, Kamstrup PR. Elevated lipoprotein(a) levels and increased risk of heart failure. 83rd Congress of the EAS, Glasgow, 22nd−25th March 2015. Abstract EAS-0432.
22. Tsimikas S et al., data in press.
23. Nordestgaard BG, Freiberg JJ, Varbo A. Extreme nonfasting remnant cholesterol vs extreme LDL cholesterol as contributors to cardiovascular disease and all-cause mortality in 90,000 individuals from the general population. 83rd Congress of the EAS, Glasgow, 22nd−25th March 2015. Abstract EAS-0450.