ADP-receptor blockage
ADP is a powerful stimulant of the platelet and acts via a specific purinoreceptor on the platelet surface. Release of ADP from dense granules is an important mechanism for positive feedback activation and recruitment of further platelets, so blockade of this pathway causes a reduction in platelet activity in response to a wide variety of stimuli. Ticlopidine, an earlier ADP inhibitor, is no longer used due to its haematological side effects. Three other drugs are currently available.
Clopidogrel
Clopidogrel is a thienopyridine derivative that is metabolised through cytochrome P450 in the liver. It dramatically inhibits platelet aggregation induced by the binding of ADP to its P2Y12 purinoreceptor on the platelet surface, a mechanism which appears to be independent of cyclooxygenase. The peak action on platelet function occurs after several days of oral dosing, and adverse effects include evidence of bone marrow suppression, in particular leucopenia.
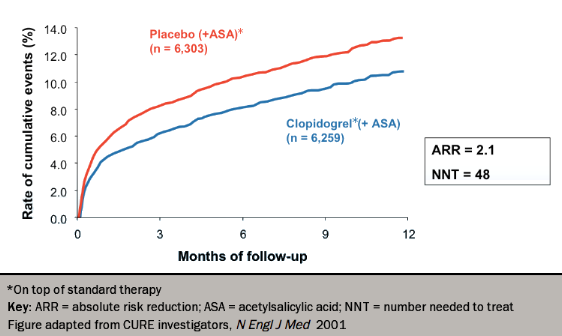
Early trials of clopidogrel in cardiovascular disease, such as CAPRIE (Clopidogrel Versus Aspirin in Patients with Atherothombosis) and CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events),13,14 showed better outcomes in combination with aspirin compared with aspirin alone, a result widely confirmed in other settings (see figure 2). However, the CASPAR (Clopidogrel and Acetylsalicylic Acid in Bypass Surgery for Peripheral Artery Disease) trial15 found no benefit of the addition of clopidogrel to aspirin in below-knee bypass grafting. Following the results of trials, such as the CURRENT–OASIS 7 (Clopidogrel and Aspirin Optimal Dose Usage to Reduce Recurrent Events − Seventh Organization to Assess Strategies in Ischemic Syndromes)16 this dual therapy is now recommended by NICE for post-acute coronary syndrome non-ST-elevation MI (NSTEMI) for 12 months, and post ST-elevation MI (STEMI) for at least four weeks after the infarction. A loading dose of 300–600 mg is generally followed by 75 mg daily.
A problem with clopidogrel follows from its prodrug status. It needs to be activated in the liver by cytochrome P450 enzymes, including CYP2C19. There are several isoforms of this enzyme: some confer loss of function (of which the most common is CYP2C19*2, possibly leading to clopidogrel resistance), while others (such as CYP2C19*17) cause a gain of function. Patients who carry one or two reduced function polymorphisms in this enzyme have been shown to be at risk of adverse cardiovascular outcomes, including stent thrombosis, leading to a Food and Drugs Administration (FDA) warning. However, a meta-analysis concluded that there is no consistent influence of CYP2C19 gene polymorphisms on the clinical efficacy of clopidogrel, and that the current evidence does not support the use of individualised antiplatelet regimens guided by CYP2C19 genotype.17 This may be because CYP2C19 genotype is only one of a number of factors which influence risk for further events in patients treated with clopidogrel.18
Another issue is that there is evidence that the use of proton-pump inhibitors (PPIs) (to reduce dyspepsia and gastrointestinal bleeding, which can be a significant problem in patients taking antiplatelet agents) reduces the antiplatelet effects of clopidogrel, most likely by inhibition of the CYP2C19 enzyme. Many local guidelines will thus advise avoidance of PPIs – especially omeprazole and esomeprazole – in patients taking clopidogrel. However, a recent meta-analysis concluded that the clinical impact of this interaction is probably not significant, pointing out that PPIs offer significant protection from gastrointestinal bleeding.19 Pending a definitive answer to this question, local policies should be followed.
