Prasugrel
Prasugrel, like clopidogrel, is a thienopyridine prodrug that is metabolised partly in the plasma by an esterase and partly via the liver cytochrome P450 system to its active metabolite, which irreversibly inhibits the platelet P2Y12 receptor. The CYP2C19 enzyme appears to have a minor role in prasugrel metabolism and the drug’s onset of action is more rapid (within 30 minutes) and consistent than that of clopidogrel.20
Given this, one would expect that prasugrel would exert greater inhibition on platelet function that clopidogrel; this does seem to be the case, and this seems to be reflected in clinical outcomes, with fewer thrombotic events but more bleeding complications in patients on prasugrel. TRITON-TIMI (Trial to Assess Improvement in Therapeutic Outcomes by Optimising Platelet Inhibition with Prasugrel – Thrombolysis in Myocardial Infarction)21 compared prasugrel and clopidogrel in over 13,000 patients, finding prasugrel to be associated with reduced cardiovascular death, non-fatal MI and stent thrombosis (HR 0.81), but more bleeding (HR 1.32). As the number of bleeds was smaller than the number of ischaemic events, there was felt to be an overall clinical benefit, although no significant mortality benefit was demonstrated.
By contrast, a second major trial, the phase III TRILOGY ACS (Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes) study compared the effect of prasugrel (10 mg daily, or 5 mg daily in patients >75 years) with that of clopidogrel (75 mg daily)22 Over 7,000 acute coronary syndrome patients under 75 years with unstable angina or NSTEMI, managed without revascularisation and taking aspirin, were followed for up to 30 months. The primary end point of the trial was cardiovascular death, MI or stroke. The study was performed at 966 sites in 52 countries.
Results showed that, through a median follow-up period of 17 months, the primary end point occurred in 13.9% of those treated with prasugrel and 16.0% of those treated with clopidogrel (hazard ratio 0.91; 95% confidence interval [CI] 0.79–1.05; p=0.21). Thus, the first trial to study the effect of platelet inhibition in patients with acute coronary syndrome managed medically without revascularisation found no significant difference between prasugrel and clopidogrel in the prevention of death, MI or stroke. However, the pre-specified analysis of multiple recurrent ischaemic events (all components of the primary end point) suggested a lower risk for prasugrel among patients under the age of 75 years (hazard ratio 0.85; 95% CI 0.72–1.00; p=0.04). Rates of severe and intracranial bleeding were similar in the two groups in all age groups.
Ticagrelor
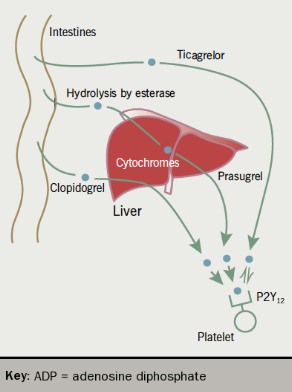
Ticagrelor is a cyclo-pentyl-triazolo-pyrimidine and is a direct and reversible P2Y12 antagonist, with a short half-life that requires twice-daily dosing, generally with a 90 mg tablet. Unlike clopidogrel and prasugrel, it is not a prodrug but acts directly and rapidly. The PLATO (Platelet Inhibition and Patient Outcomes) trial23 compared ticagrelor with clopidogrel in patients with STEMI, or moderate to high risk NSTEMI. Ticagrelor reduced the risk of a composite outcome of death from vascular causes, MI, or stroke (HR 0.84). There was no significant increase in major bleeding rates with ticagrelor overall, but there was a small increase in the risk of non-procedure related bleeding, including intracranial haemorrhage.
Apart from bleeding, side effects associated with ticagrelor include elevated creatinine concentrations, increased ventricular pauses, and dyspnoea (11.8% in PLATO study).
Cangrelor
Cangrelor is an intravenous ATP analogue, which provides reversible P2Y12 inhibition with rapid onset and offset (within one to two hours) of action. A recent meta-analysis of the three CHAMPION trials of cangrelor initiated at the beginning of percutaneous coronary intervention (PCI) versus clopidogrel, showed a 19% relative risk reduction in the primary end point of periprocedural death, MI, ischaemia-driven revascularisation and stent thrombosis, with a small increase in bleeding.24 It received marketing authorisation in the EU in 2015.
The differences in the metabolism of the three oral ADP-receptor blockers are summarised in figure 3.
Choosing an ADP-receptor blocker: a role for platelet function testing?
Prasugrel and ticagrelor both seem more effective than clopidogrel at preventing cardiovascular events but at a cost of increased bleeding risk. They are also considerably more expensive than clopidogrel, which is now off patent. Are there any laboratory tests which help us to decide which agent to prescribe for an individual patient?
We have seen that testing for CYP2C19 status has not yet gained universal acceptance as helpful in this context. Another approach has been to measure the reactivity of platelets following antiplatelet administration, with a view to switching therapy for those patients whose platelets do not seem adequately suppressed.
Reference 25 has a detailed account of these tests and their clinical application.
VerifyNow® P2Y12 Assay (Accumetrics, USA)
This is perhaps the most widely used assay; it is quick and simple to use, and its small size makes it suitable for bedside testing. Whole blood is dispensed into a test cartridge containing ADP and fibrinogen-coated beads. Activated platelets aggregate and agglutinate on these beads, and this process is measured by light transmission through the sample. Light transmittance units are then converted into P2Y12 reaction units (PRUs). A consensus definition of high on-treatment platelet reactivity is a PRU >208.
Multiple electrode aggregometry (Multiplate, Roche Diagnostics, Switzerland)
This is a laboratory aggregometer, which measures platelet aggregation in whole blood by electrical impedance. It is only partially automated, therefore requiring the availability of trained laboratory staff.
Flow cytometric analysis of VASP phosphorylation
Vasodilator stimulated phosphoprotein (VASP) is a second messenger in the signalling pathway from the P2Y12 receptor. Stimulation of this receptor leads to dephosphoryation of VASP and this can be measured by flow cytometry. This measurement is highly specific for the P2Y12 pathway but is a technically difficult test requiring highly skilled staff.
There is accumulating evidence that high on-treatment platelet reactivity, as measured by these tests, is associated with adverse cardiovascular outcomes – at least for patients with acute coronary syndrome undergoing PCI. There is, however, not yet any good trial evidence that switching therapy based on the results of testing improves outcomes.18
In medically managed patients, and chronic ischaemic heart disease, there is not even any good evidence that platelet function testing adds any prognostic information above that provided by conventional risk factor assessment. In the TRILOGY ACS study, for example, high platelet reactivity by VerifyNow® was not an independent predictor of adverse events.18 The TRIGGER-PCI (Testing Platelet Reactivity in Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy with Prasugrel) trial enrolled 423 patients undergoing elective PCI with drug-eluting stent placement. All had platelet reactivity measured following clopidogrel administration; patients with an inadequate response were randomised to prasugrel or to continued clopidogrel. Although prasugrel was demonstrably more effective than clopidogrel at reducing platelet reactivity in these patients, this did not translate into statistically different outcomes. The event rate was, however, very low in both arms, limiting the usefulness of this study.26
Some smaller and non-randomised studies have reported data supporting a benefit for treatment changes based on platelet function tests, particularly in high-risk patients.18 Moreover, low on treatment platelet reactivity may be associated with increased bleeding risk and could be used to plan surgery in patients taking antiplatelet agents.18 The role of these tests may therefore increase in the future.
ADP receptor blockers – current guidelines
In the absence of agreement on the place of platelet testing, guidelines on ADP-receptor blocker choice are largely based on the available trial evidence.
NICE guidance on prasugrel (TA18227) is based on a detailed analysis of the TRITON-TIMI trial. Prasugrel is recommended as an option, in addition to aspirin, in acute coronary syndromes requiring PCI, only when: (i) immediate PCI for STEMI is required; or (ii) there is stent thrombosis in patients on clopidogrel; or (iii) in patients with diabetes.
For ticagrelor, NICE guidance (TA23628) recommends its use for up to 12 months, alongside low-dose aspirin, after an acute coronary syndrome (STEMI plus primary PCI, NSTEMI, or admission with unstable angina).
Recent ESC Guidelines recommend ticagrelor or prasugrel first line along with aspirin in patients with STEMI. For NSTEMI, ticagrelor is recommended for all patients at moderate to high risk of ischaemic events, with prasugrel as an option for those undergoing PCI. Clopidogrel is relegated to second line, for patients who can not receive either of the other agents, or who also require oral anticoagulation (where the bleeding risk with prasugrel or ticagrelor is felt to be unacceptably high).29,30
Clearly this has significant cost implications – local guidelines should be followed.
Preventing platelet–platelet interactions
GpIIb/IIIa, also known as integrin αIIbβ3 is the most important platelet surface receptor in achieving stable platelet aggregation. It binds fibrinogen, and other platelets via fibrinogen. It is the most abundant glycoprotein on the platelet surface, and its numbers and adhesive properties are increased by platelet activation of any cause (inside-out signalling). Binding to GpIIb/IIIa also contributes to platelet activation (outside-in signalling). The central importance of GpIIb/IIIa to platelet function is demonstrated by the severe bleeding phenotype associated with its congenital absence (Glanzmann’s thrombasthenia).
For all these reasons GpIIb/IIIa is an attractive target for critical occasions when profound platelet inhibition is required.Three GpIIb/IIIa inhibiting agents are available, each of which must be given by injection or infusion. They are NICE approved for the early management of high-risk patients with acute coronary syndromes for whom early PCI is planned; their use should be by specialists only.31
Abciximab
Abciximab has a long history, being first in its class, not only in GpIIa/IIIb blockage but also as a therapeutic monoclonal antibody. It is an established agent in the prevention of aggregation in acute coronary settings (alongside heparin and aspirin) and inhibits aggregation by 90% within two hours of its infusion. Platelet function then recovers over the course of two days but it has a major adverse effect of haemorrhage. There should be caution in its use in severe renal impairment. Abciximab can cause thrombocytopaenia within two to four hours of commencement. This can rarely be severe (<20 x 109/L).
Eptifibatide
Eptifibatide is a cyclic heptapeptide that mimics the part of the structure of fibrinogen that interacts with GpIIb/IIIa. Thus, it is a fraction of the size of abciximab and is targeted at the same structure on the platelet surface. It is licensed for the prevention of early MI in patients with unstable angina or NSTEMI. There is again caution in renal impairment, with a reduction in dose if estimated glomerular filtration rate (eGFR) <50 ml/min/1.73 m2, and should be avoided if eGFR <30 ml/min/1.73 m2.
Tirofiban
The third agent, tirofiban has a similar licence and contraindications as eptifibatide, including abnormal bleeding, severe hypertension (as this is a risk factor for haemorrhagic stroke), use of oral anticoagulants and hepatic impairment. However, the level of caution in renal disease is eGFR <60 ml/min/1.73 m2, with the use of half the dose when eGFR <30 ml/min/1.73 m2.
