Oral anticoagulants
Warfarin
Warfarin inhibits the enzyme vitamin K epoxide reductase. This prevents the recycling of vitamin K, which is essential for carboxylation of several clotting factors. Without this carboxylation they will not function. Factors affected by warfarin are factors II, VII, IX and X; it also reduces levels of protein C and protein S. For those in whom warfarin is contra-indicated, perhaps by intolerance or allergy, alternative VKAs are available: acenocoumarol and phenidione.
Warfarin is a long-established and effective anticoagulant, which is safe in renal impairment, and has established reversal agents available (prothrombin complex concentrate for emergency reversal, vitamin K for reversal over hours). However, there are a number of problems associated with its use, including its numerous drug interactions, a narrow therapeutic window with requirement for frequent monitoring blood tests, and the long time (days) taken to reach a therapeutic level. Practical issues relating to warfarin use are discussed in modules 4 and 5.
Non-vitamin K antagonist oral anticoagulants (NOACs)
Previously known as novel or new oral anticoagulants, not all these agents are really new anymore (dabigatran obtained its US licence in 2010). This has led to them being renamed, e.g. by NICE as non-vitamin K antagonist oral anticoagulants. The International Society for Thrombosis and Haemostasis, however, recently suggested a change of name to DOACs,6 in recognition of their mode of action: direct inhibition of one clotting factor, rather than interference with the metabolism of several, as warfarin does.
In fact the grouping is somewhat false, as the agents do not share the same mechanism of action: three are inhibitors of factor Xa (rivaroxaban, apixaban and edoxaban) while dabigatran inhibits thrombin. What they share, however, are rapid onset of action from oral administration, and predictable pharmacokinetics such that monitoring of anticoagulant effect is not routinely required.
Discussion of drug dosing below is for the atrial fibrillation indication. Always consult up to date product literature or the British National Formulary for prescribing information.
Dabigatran
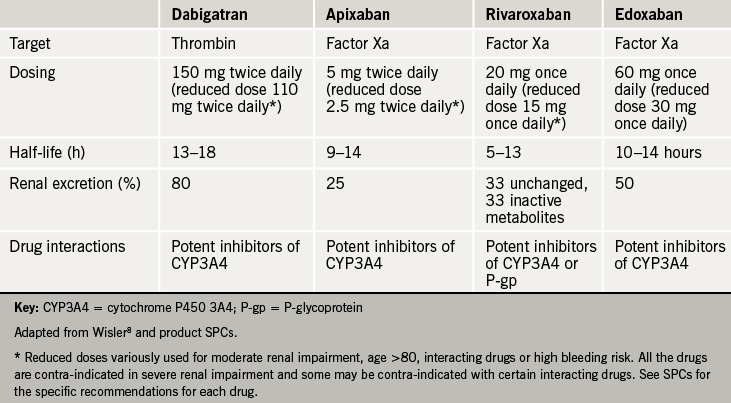
Dabigatran is an oral direct thrombin inhibitor. It has rapid absorption (within two hours) and distribution with an estimated half-life of 13–18 hours. Nearly 80% is excreted unchanged by the kidneys, so that half life can be significantly prolonged in renal impairment, and dabigatran is contraindicated in severe renal impairment (creatinine clearance <30 ml/min).
Although drug interactions are few, dabigatran is a substrate for the efflux transporter P-glycoprotein (P-gp), so that levels of dabigatran are increased by P-gp inhibitors. Some of these interactions are potentially highly significant in cardiac patients: strong P-gp inhibitors (ketoconazole, cyclosporine, itraconazole and dronedarone) are contraindicated with dabigatran, while moderate inhibitors (amiodarone, verapamil, quinidine, clarithromycin and ticagrelor) should be used with caution.
A reduced dose is suggested by the manufacturer for elderly patients, patients with renal impairment, patients on one of the drugs listed above, or other patients felt to be at high risk of bleeding.
Rivaroxaban
This was the first selective oral direct factor Xa inhibitor on the market.7 It inhibits both free and clot bound factor Xa, and, unlike heparin, does not depend on antithrombin for its action. It achieves peak plasma levels in two hours and has a half-life of 5–13 hours – longer in elderly subjects and those with renal impairment. Rivaroxaban is dosed once daily.
Excretion is approximately one third renal – a dose reduction is recommended with creatinine clearance 15–49 ml/min, and the drug contraindicated below 15 ml/min. A further one third of rivaroxaban is metabolised by the liver via CYP3A4, with the final third excreted through the gut via P-gp. Inhibitors of both CYP3A4 and P-gp might therefore be expected to raise rivaroxaban levels, and certain drugs (such as azole antifungals, and HIV protease inhibitors) are contrainidicated. Full information can be found in the rivaroxaban SPC.
Apixaban
Apixaban’s mechanism of action and metabolism is much as for rivaroxaban, except that, although its half life is between 9–14 hours it is dosed twice daily. It has the least dependence on renal excretion of the NOACS at 25%, but shares rivaroxaban’s dependence on metabolism by CYP3A4.
The manufacturer recommends a dose reduction for patients with creatinine clearance 15–29 ml/min, and also for patients with serum creatinine ≥1.5 mg/dL (133 micromol/l) associated with age ≥80 years or body weight ≤60 kg. Apixaban is contraindicated at creatinine clearance <15 ml/min – see SPC for further details.
Edoxaban
Another inhibitor of factor Xa, Edoxaban is newly licensed in Europe, but has been licensed in Japan since 2012. It is dosed once daily, with a half life of 10–14 hours. 50% is renally excreted, with the rest excreted via P-gp – it has the least CYP3A4 interaction of all the Xa inhibitiors. As for rivaroxaban, dose reduction is recommended at 15–49 ml/min, and the drug contraindicated below 15 ml/min. A lower dose is also recommended for patients <60 kg, and those taking P-gp inhibitors.
The relevant profiles of these four NOACs are shown in table 2.8