Warfarin
Warfarin’s efficacy and safety depend crucially on how well-controlled the INR is (see module 4). NICE advise regular review, and consideration of switching to a DOAC if the time in therapeutic range (TTR) is <65%.
Dabigatran
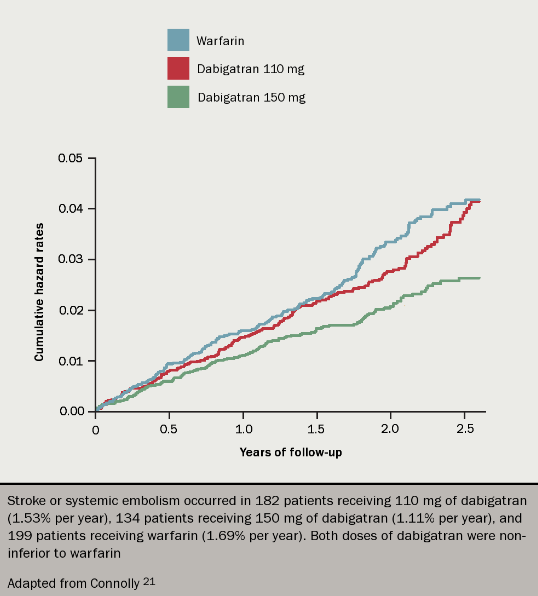
Dabigatran was compared to warfarin for the prevention of stroke in AF in the RELY trial (see figure 4).21 Over 18,000 patients received 110 mg or 150 mg of dabigatran twice a day, or warfarin to target INR 2.5. Yearly rates of the primary outcome (stroke or systemic embolus) were 1.69% in the warfarin group, and 1.53% and 1.11% in the dabigatran 110 mg and 150 mg groups respectively, the latter being significantly lower than the warfarin result.
The rate of the primary side effect of major bleeding was 3.36%, 2.71% and 3.11% respectively. Therefore dabigatran 110 mg bd was equally as efficacious as warfarin but had a better safety profile in terms of major bleeding, whereas the 150 mg dose twice a day was superior to warfarin in terms of stroke prevention but had similar rates of bleeding. Closer scrutiny of the trial data shows that rates of intracranial haemorrhage were lower with dabigatran even at the higher dose. The higher dose of dabigatran was, however, associated with significantly more gastrointestinal bleeds than warfarin.
One other result of concern was a small increase in rates of myocardial infarction in both dabigatran groups, which was statistically significant for the 150 mg dose. This was, however, not replicated in a real-world post-marketing study conducted by the US FDA.22
Rivaroxaban
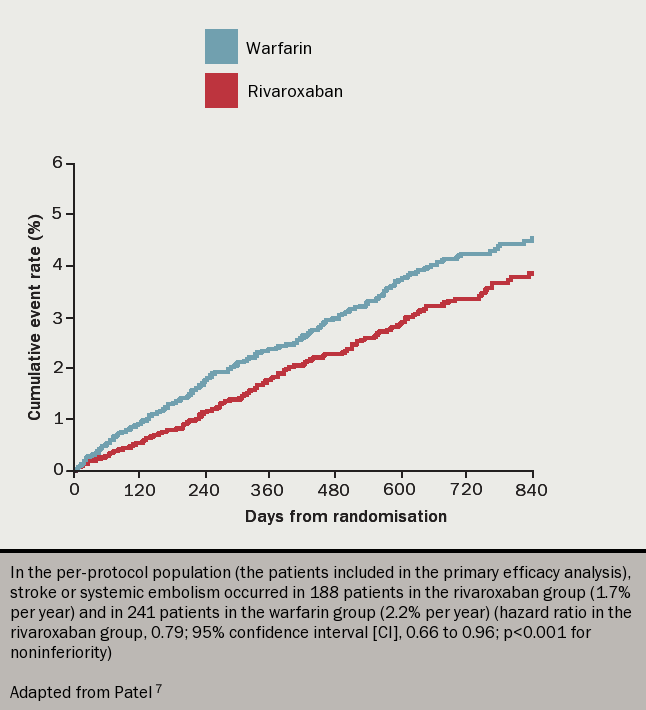
Rivaroxaban was also compared to warfarin for the prevention of stroke in AF. The ROCKET-AF7 trial (see figure 5) randomly assigned over 14,000 patients to rivaroxaban 20 mg once daily or to dose-adjusted warfarin, finding rates of stroke or systemic embolism to be 1.7% and 2.2% respectively, whilst the major and minor bleeding events were 14.9% and 14.5% respectively (all differences not significant). However, there were significantly fewer fatal bleeds and cases of intracranial haemorrhage in those taking rivaroxaban, but again more gastrointestinal bleeding.
Apixaban
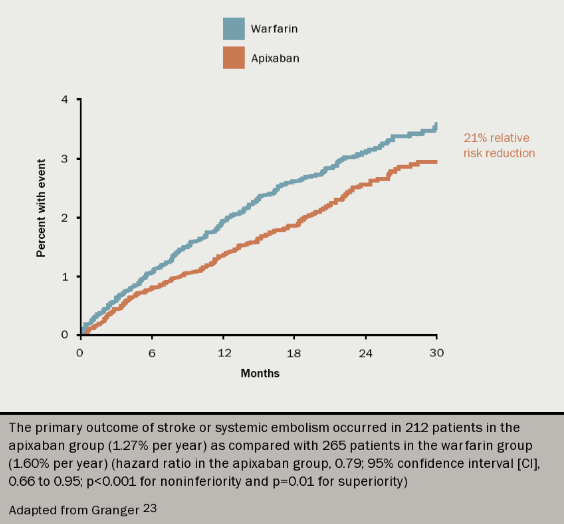
In the ARISTOTLE trial (see figure 6),23 apixaban was compared to warfarin for stroke prevention in over 18,000 patients with AF and at least one additional risk factor for stroke. After 1.8 years of follow up, yearly rates of the primary end point (any stroke, or systemic embolism) were 1.27% on apixaban and 1.6% on warfarin (p=0.01). Furthermore, apixaban was associated with significantly fewer cases of major bleeding and fewer haemorrhagic strokes (both p<0.001). Rates of gastrointestinal bleeding were the same.
Edoxaban
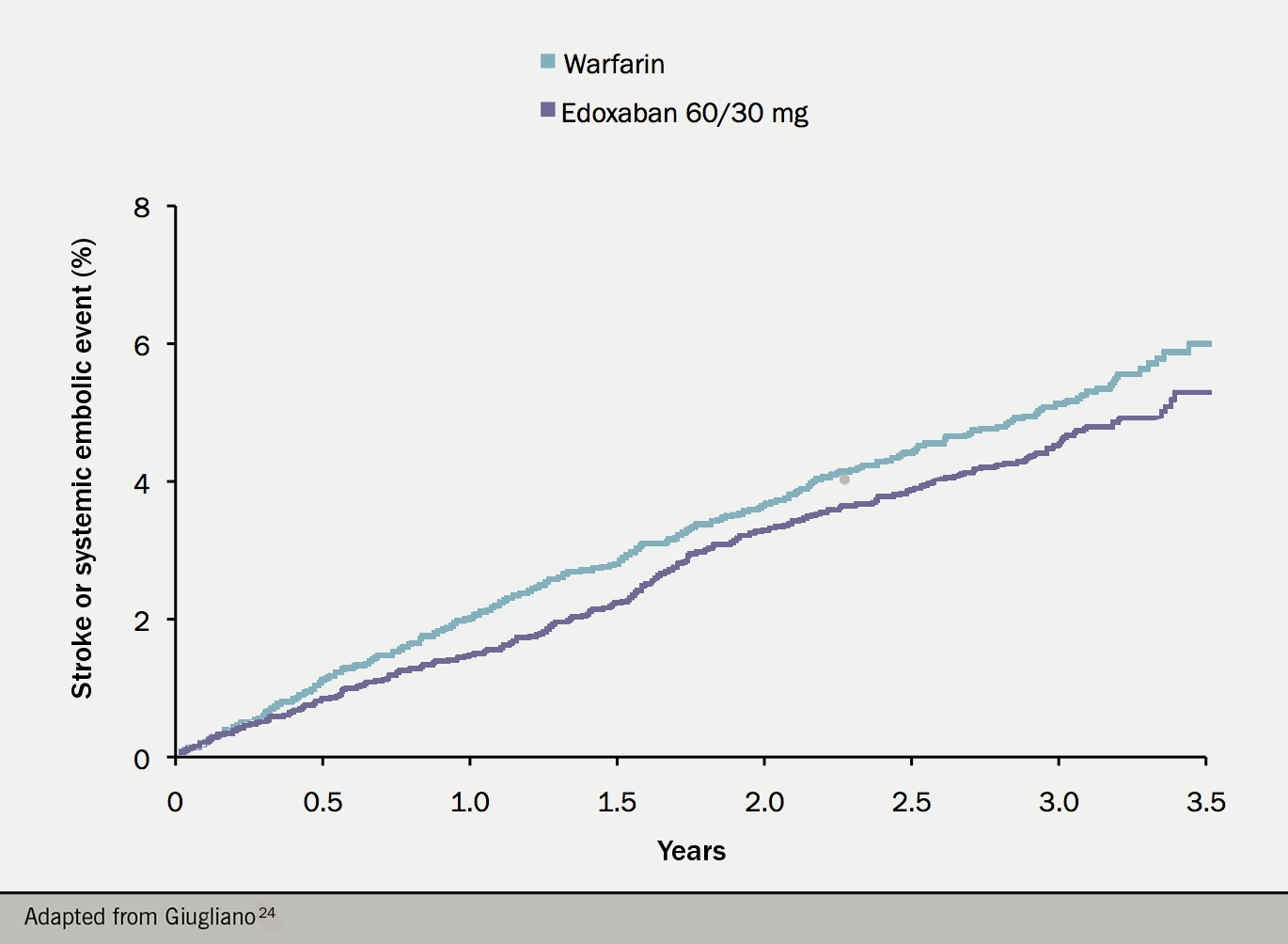
The ENGAGE AF-TIMI 48 trial (see figure 7) randomised over 21,000 patients with non-valvular AF and a CHADS2 score of 2 or more to one of two dose regimens of edoxaban, or warfarin. The higher dose regimen showed a trend towards superiority over warfarin in terms of the primary outcome (any stroke or systemic embolus). Bleeding (including major, life-threatening and intracranial bleeding) was significantly lower with edoxaban, with the exception of gastrointestinal bleeding which was significantly more common.24 Based on these results, the higher dose regimen was licensed: 60 mg once daily is the standard dose, with 30 mg used for patients with low weight, renal impairment, or on potent P-gp inhibitors.
