A. Menarini Farmaceutica Internazionale SRL has provided an educational grant for the production of this e-learning programme and has had no editorial control or input. The views and content expressed within this programme are solely those of the authors.
PP-CA-UK-0159 Date of preparation: March 2020
The revascularisation option in stable angina
Various European,1 UK2 and US3–7 guidelines have been published on the use of revascularisation, i.e. percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG), in patients with occlusive coronary artery disease (CAD) (see practice point below). In most European countries, PCI now accounts for at least 70% of all revascularisations, although elective PCI for stable CAD has decreased.8,9 Nevertheless, PCI has become one of the most frequently performed therapeutic interventions in medicine, and progress has resulted in a steady decline of peri-procedural adverse events, resulting in excellent outcomes.1
General recommendations on revascularisation
The UK National Institute for Health and Care Excellence (NICE) guidance on the treatment of stable angina2 highlights, in its list of key priorities for implementation:
- the use of revascularisation (CABG or PCI) for patients whose symptoms are not satisfactorily controlled using optimal medical treatment (OMT).
Practice point. Indications for revascularisation in stable angina or silent ischaemia1
| For prognosis | Left main disease with stenosis >50%** |
|---|---|
| Any proximal LAD stenosis >50%** | |
| Two-vessel or three-vessel disease with stenosis >50%* with impaired LV function (LVEF <35%)** | |
| Proven large area of ischaemia (>10% LV) or abnormal invasive FFR (iFFR)* | |
| Single remaining patient coronary artery with stenosis >50%** | |
| For symptoms | Any coronary stenosis >50%** in the presence of limiting angina or angina equivalent, unresponsive to medical therapy |
| Key: * = FFR < 0.75; ** = with documented ischaemia or FFR ≤0.80 or iwFR ≤0.89; FFR = fractional flow reserve; iFFR = invasive fractional flow reserve; iwFR = instantaneous wave-form ratio; LAD = left anterior descending; LV = left ventricle; LVEF = left ventricular ejection fraction Adapted from Neumann FJ1 |
|
When either procedure could be appropriate, the guidance recommends that it is important to explain to the patient “the risks and benefits of PCI and CABG for people with anatomically less complex disease whose symptoms are not satisfactorily controlled with (OMT)”. If the patient does not express a preference, NICE points to evidence suggesting that “PCI may be the more cost-effective procedure in selecting the course of treatment”.
However, NICE recommends considering the potential survival advantage of CABG over PCI for patients with multivessel disease whose symptoms are not satisfactorily controlled with OMT, and who:
- have diabetes
- are over 65 years or
- have anatomically complex three-vessel disease, with or without involvement of the left main stem.
There is much interest in which patients are likely to benefit more from PCI, or from CABG surgery. Figure 1 shows a flow-chart of revascularisation strategies in chronic stable angina.10 As PCI techniques and devices develop, e.g. drug-eluting stents (DES), the distinction between these two treatment options for patients has become less clear. Although CABG has, to date, been the preferred therapy for left main disease, recently published results of the EXCEL (Evaluation of XIENCE versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization) trial suggest that PCI is as good as CABG, with similar rates of adverse cardiac outcomes, in patients with left main CAD of low or intermediate anatomical complexity.11 As we go to press, this finding is the subject of much controversy.
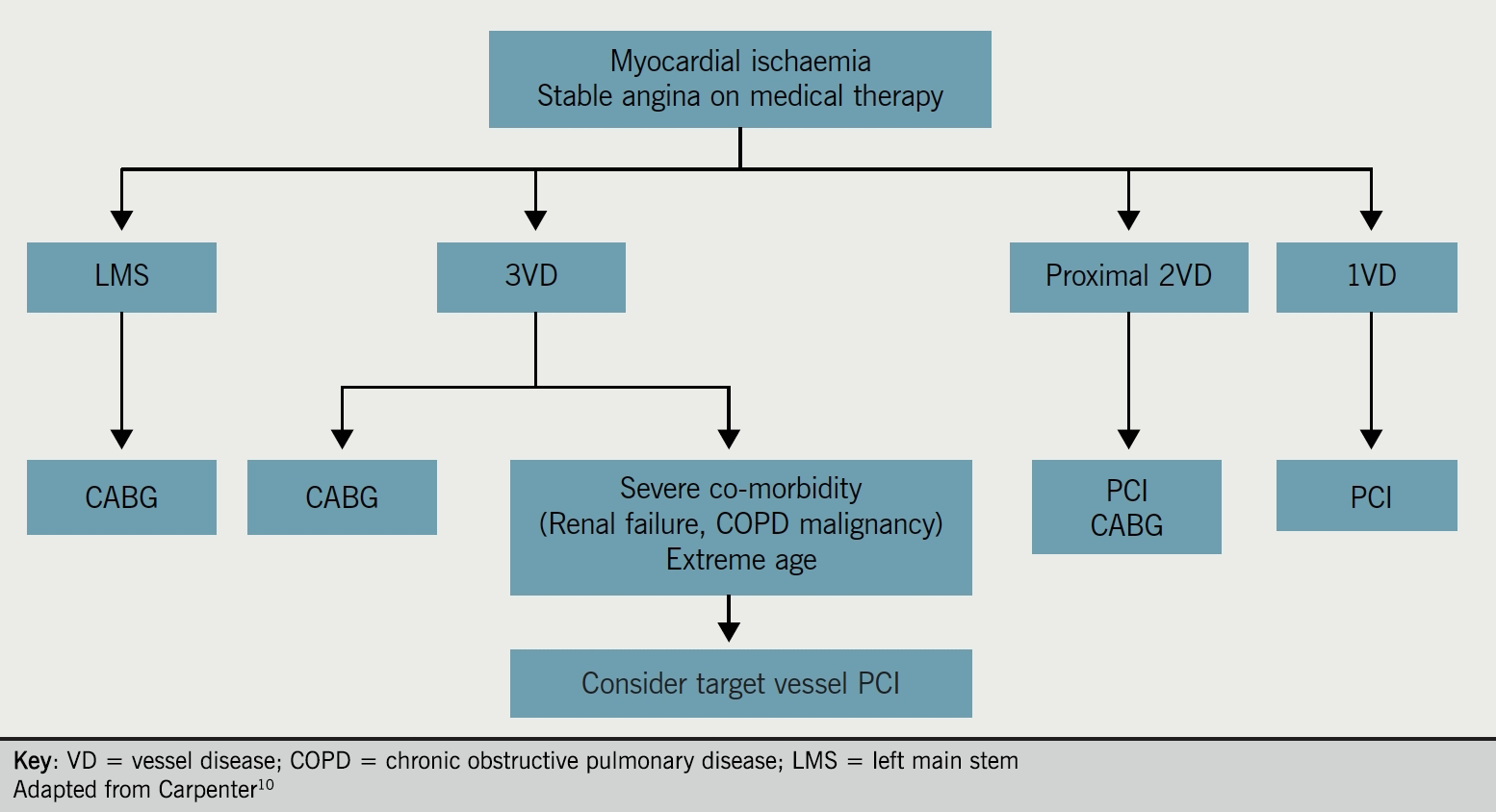
Table 1. Areas for discussion to involve patients in decision-making on revascularisation
| The prognostic benefit for left main stem or triple-vessel disease, particularly in the context of impaired left ventricular function, particularly with CABG |
| The relief of angina symptoms with CABG and PCI both effective in relieving symptoms |
| Repeat revascularisation may be necessary after either CABG or PCI. The rate is lower after CABG |
| Stroke is uncommon after either CABG or PCI and incidence is similar between the two |
Practical aspects of the procedures
|
| Key: CABG = coronary artery bypass graft; PCI = percutaneous coronary intervention |
When deciding appropriate revascularisation strategies, the importance of a heart team discussion should be emphasised.12 This should include cardiac surgeons and interventional cardiologists. NICE recommends discussion of, but not limited to:
- left main stem or anatomically complex triple-vessel disease
- cases where there is doubt about the optimal method of revascularisation due to:
- complexity of coronary anatomy
- extent of stenting required
- other relevant clinical factors and co-morbidities.
It is important to involve patients in the decision-making process, provide them with balanced information, and ensure they are appropriately counselled. Areas for discussion are shown in table 1.
In summary
Coronary revascularisation should be considered in patients with stable angina pectoris who either remain symptomatic despite or intolerant of OMT. High-risk patient groups with stable CAD who may especially derive benefit from coronary artery bypass grafting (CABG) include those with:
- lesions involving the left main stem or proximal left anterior descending (LAD) coronary arteries, particularly where there is left ventricular systolic dysfunction.
- a large territory of reversible ischaemia demonstrated on functional testing.
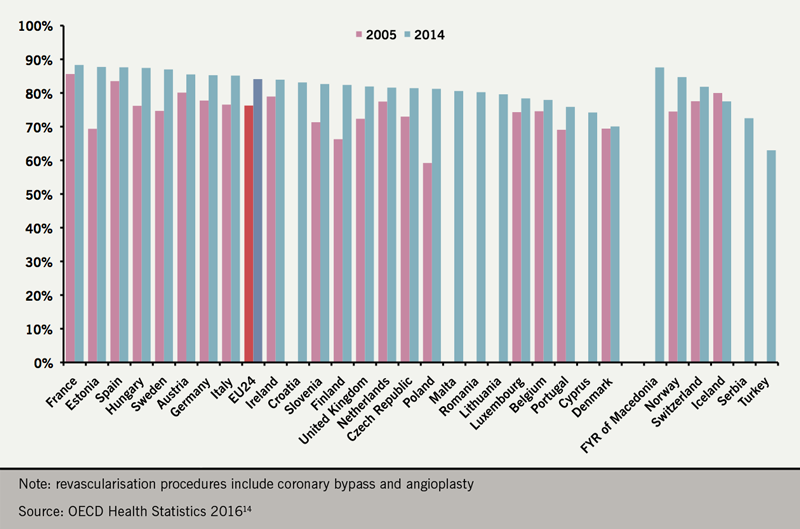
In the UK, 102,258 PCI and 14,731 CABG procedures were performed in 2017 (figure 2). Approximately 33% of the PCI procedures were performed on patients with stable CAD.13 Recent data point also towards a wide variability in PCI rates throughout Europe (figure 3), although the variability is lessening.14
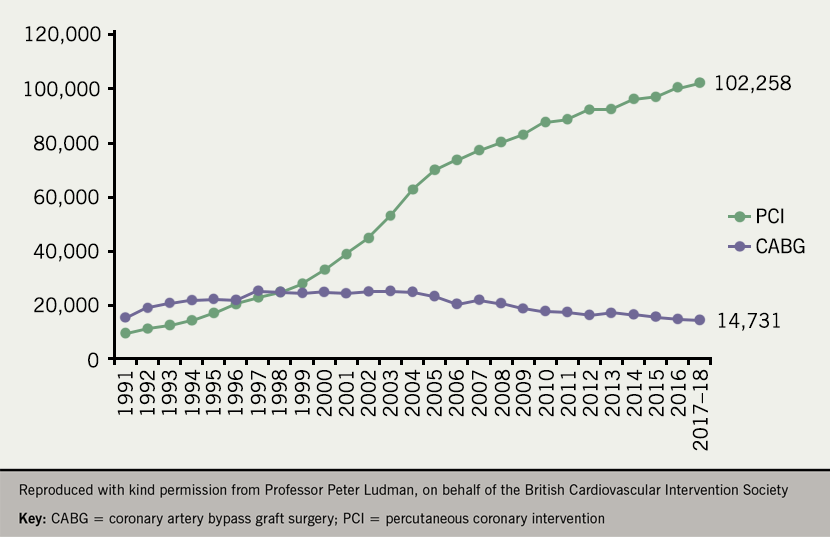
US studies have shown a steady decline in annual CABG utilisation rates between 2001 and 2008 despite an aging population, whilst there was no change in the rates of PCI.15 Of note, there has been nearly a 30% decline in CABG procedures in the US over the past decade.16
Trials, including COURAGE, and their implications
Although PCI is highly effective and superior to medical therapy for relief of symptoms15 and ischaemic burden17 in patients with stable CAD, it does not appear to provide additional reduction of the risk of either death or recurrent myocardial infarction (MI) in this patient group.18 The COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial conducted in North America, found that PCI provides no benefit to outcomes for CAD patients over OMT (figure 4).18
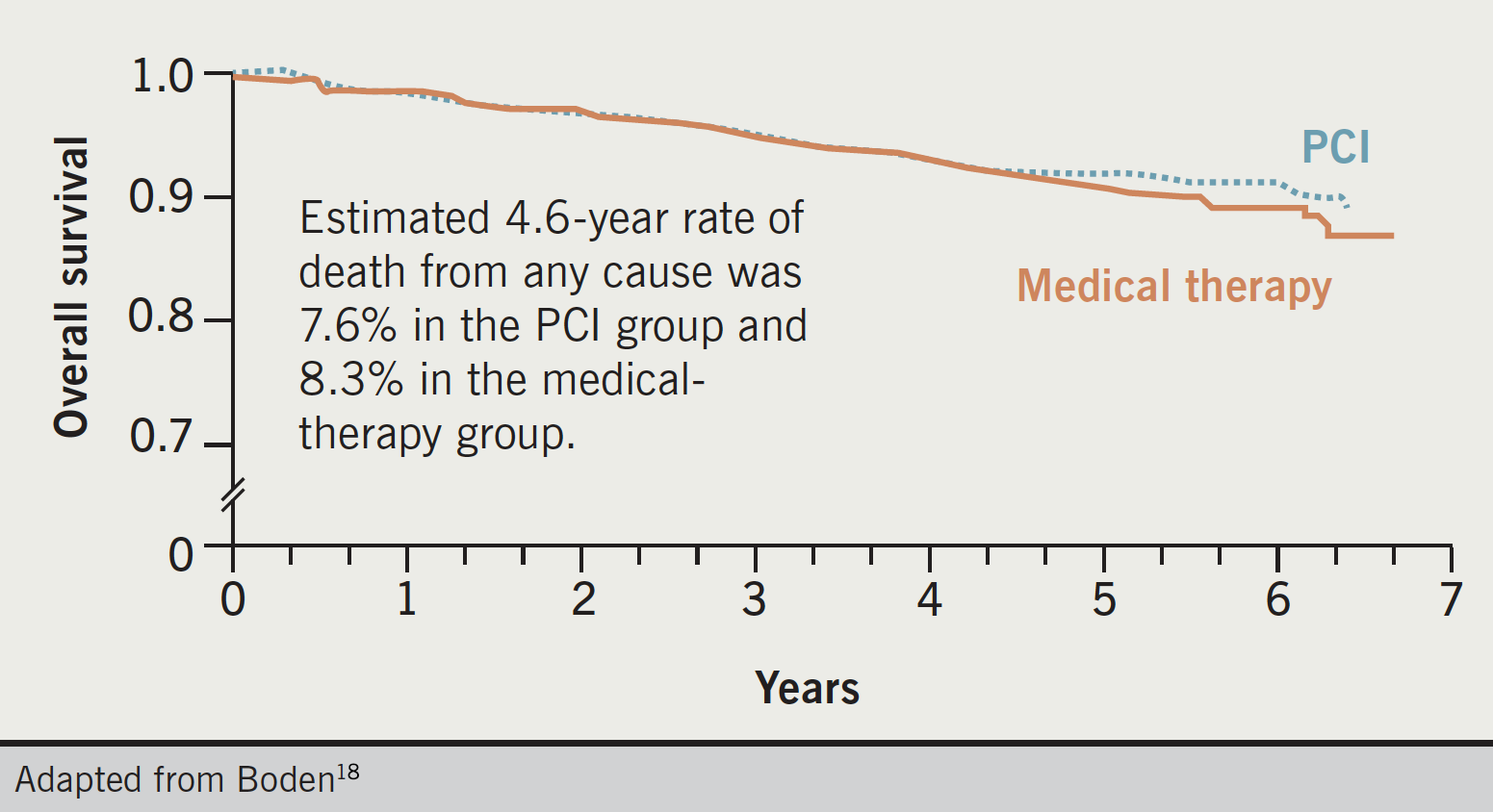
Researchers randomised 2,287 patients with known significant CAD and objective evidence of myocardial ischaemia to OMT alone or to OMT and PCI. At a median follow-up of 4.6 years, there was no significant difference in the composite end point of death, MI, stroke, or hospitalisation for unstable angina. Initially, the PCI group displayed greater freedom from angina during follow-up, but the gap narrowed over five years with 74% of patients in the PCI group and 72% of those in the OMT group free of angina after that time.
The authors concluded that an initial strategy of PCI in stable CAD did not reduce the risk of death, MI, or major adverse cardiac events when added to OMT. Their findings support existing clinical practice guidelines, which state that “PCI can be safely deferred in patients with stable coronary artery disease,” even in those with extensive, multivessel involvement and inducible ischaemia, “provided that intensive, multifaceted medical therapy is instituted and maintained”.18
This conclusion is partially supported by findings from a European study on the long-term outcomes of 730 patients with an angiographically determined left anterior descending coronary artery (LAD) stenosis, given either medical treatment or revascularisation depending on fractional flow reserve (FFR) measurements.19 After a median follow-up of 40 months, researchers found that medical treatment of patients with a haemodynamically non-significant stenosis in the proximal LAD was associated with excellent long-term clinical outcome, with survival at five years similar to an age- and sex-matched control population. The authors state that their study “supports the strategy of deciding about revascularisation based on both anatomic and functional information obtained simultaneously in the catheterisation laboratory”. However, the deferral of PCI in this study was based on the presence of non-flow limiting epicardial CAD in contrast to what the authors concluded in the COURAGE trial.
Study limitations
Both studies however have limitations. The COURAGE trial enrolled only 6.4% (2,287/35,539) of the patients evaluated,18 raising questions about its general applicability. Among others, low ejection fraction (<30%), a markedly positive stress test, and unprotected left main disease were noteworthy exclusion criteria. In addition, a third of the patients in the OMT group underwent coronary revascularisation during follow-up, which can be considered a relevant, though appropriate, cross-over rate.
The second study basically confirmed previous findings of the randomised controlled DEFER trial (stable patients scheduled for PCI without documented ischaemia).20 Nevertheless, it should be pointed out that patients who did not undergo PCI had an intermediate stenosis (between 30% and 70% by visual estimate) and absence of ischaemia in the LAD territory.19 Revascularisation of these lesions appears definitely not appropriate.
Interestingly, a substudy of selected COURAGE patients showed that adding PCI to OMT resulted in greater reduction in inducible ischaemia compared with OMT alone and that the risk for death or MI during follow-up was proportional to the magnitude of residual ischaemia.17 Previously, the small SWISSI II (Swiss Interventional Study on Silent Ischemia Type II) randomised trial, suggested that among patients with recent MI, silent myocardial ischaemia, and one- or two-vessel coronary artery disease, PCI compared with anti-ischaemic drug therapy reduced the long-term risk of major cardiac events.21 There are several other observational studies suggesting that revascularisation might be associated with improved clinical outcome in patients with documented moderate-to-severe myocardial ischaemia or viable myocardium, in particular when associated with left-ventricular dysfunction and multivessel CAD.22
Collectively, these findings should not discourage PCI and revascularisation when clinically appropriate, i.e. in the presence of relevant symptoms or severe ischaemia despite OMT. Conversely, there is certainly no benefit and probably a hazard associated with revascularisation of lesions that do not produce ischaemia. This had been consistently shown by many other clinical trials, such as DEFER,20 OAT23 and AVERT.
PCI using second-generation DES has been shown in a meta-analysis to confer an improved survival compared to medical treatment.25 The use of PCI to reduce symptoms and ischaemia, however, has recently been questioned and may not be as effective as once thought. The ORBITA (Objective Randomised Blinded Investigation with Optimal Medical Therapy for Angioplasty in Stable Angina) study was a sham-controlled randomised trial (n=200) that used drug-eluting stents to treat angiographically significant stenoses and found that PCI did not improve exercise time or anginal frequency at six weeks compared to sham procedures despite the presence of anatomically and functionally significant stenoses,26 even though ischaemia was more effectively treated with PCI.
This is an interesting result but there are a number of limitations to this study. Although exercise times and treadmill scores trended higher with PCI, it may have been underpowered and a larger sample size would have detected between the groups.27 If the follow-up period was extended, it may also have shown a difference in outcomes.28 Furthermore, after the study, 85% of the sham placebo group opted to have PCI, which is a very high proportion. Patients gave reasons such as difficulty taking medication and reduced exercise capacity due to taking medications.
Type 2 diabetes
The BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) study extended these findings to patients with type 2 diabetes.29 Overall, the investigators did not observe a significant difference in the rates of death and major cardiovascular events between patients undergoing early revascularisation and those in the OMT arm up to five years. The population of the BARI 2D trial however can also be considered a low-to-moderate risk population – 81.8% of the patients were asymptomatic or with mild angina (CCS class 1 and 2), only 13.2% had proximal LAD disease and there is no information about the extent of myocardial ischaemia. DES and thienopyridines (e.g. clopidogrel and ticlopidine) were used in a minority of patients. Further, at five years, there was a crossover rate of 42.1% from OMT to revascularisation. Interestingly, a post-hoc analysis showed a lower rate of major cardiovascular events in the CABG group as compared to OMT (22.4% vs. 30.5%, p=0.01; p=0.002 for interaction between stratum and study group), a difference mainly driven by a lower rate of non-fatal MI.29
The FREEDOM (Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease) trial investigated whether PCI or CABG had better outcomes in patients with diabetes and multivessel CAD.30 It found that the primary outcome (five-year rate of composite of all-cause mortality, nonfatal MI, or nonfatal stroke) was significantly better with CABG (18.7%) compared to PCI (26.6%), mainly due to reduced rates of MI and death from any cause, although notably five-year stroke risk was more than twice as frequent with CABG (5.2%) than PCI (2.4%).
Discussion
Other observations pertaining to the COURAGE trial and its effects in clinical practice are also worth mentioning. A number of registries documented suboptimal use of recommended preventive therapies outside the ideal setting of clinical trials. Disappointingly, according to recent data from a large multi-centre US PCI registry, the percentage of patients with stable CAD receiving OMT did not noticeably improve following the publication of COURAGE.31 The investigators reported that less than half of people before PCI were taking OMT, increasing to about two-thirds post-PCI; after the publication of the trial results, these practice patterns changed significantly from a statistical point of view, but the variations did not appear as “clinically relevant” (OMT before PCI in 43.5% of patients before- and 44.7% after-COURAGE; OMT at discharge 63.5% and 66.0%, respectively).31
The reasons that make it difficult to translate the results of the trial into mainstream clinical practice deserve further investigation, but it is likely these include side effects and low patient compliance to a high number of medications. Conversely, another large registry demonstrated a significant and sustained decline (~25%) in the use of PCI for stable angina after publication of the COURAGE trial,32 suggesting that clinicians did accept the main message of the study and reacted accordingly.
The ISCHEMIA trial – and its implications
The goal of the ISCHEMIA (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches) trial33 was to evaluate routine revascularisation compared with OMT in stable CAD patients with moderate to severe myocardial inducible ischaemia. Some 5,179 patients were randomised to routine invasive therapy (n = 2,588) versus medical therapy (n = 2,591).
The primary outcome of cardiovascular death, MI, resuscitated cardiac arrest, or hospitalisation for unstable angina or heart failure at 3.3 years occurred in 13.3% of the routine invasive group compared with 15.5% of the medical therapy group (p = 0.34). The findings were the same in multiple subgroups. Invasive therapy was associated with harm (~2% absolute increase) within the first six months and benefit within four years (~2% absolute decrease).
Secondary outcomes:
- cardiovascular death or MI: 11.7% of the routine invasive group vs. 13.9% of the medical therapy group (p = 0.21)
- all-cause death: 6.4% of the routine invasive group vs. 6.5% of the medical therapy group (p = 0.67)
- periprocedural MI: (invasive/conservative hazard ratio [HR] 2.98, 95% confidence interval [CI] 1.87–4.74)
- spontaneous MI: (invasive/conservative HR 0.67, 95% CI 0.53-0.83)
Discussion
The study appears to validate the importance on OMT and the need to control risk factors in stable CAD patients. Routine revascularisation failed to reduce major adverse cardiac events. There was also no benefit from revascularisation regarding all-cause mortality or cardiovascular mortality/MI. One third of subjects reported no angina symptoms at baseline. Revascularisation was associated with harm at six months (increase in periprocedural MI rates) and associated with benefit at four years (reduction in spontaneous MI). These results do not apply to patients with current/recent acute coronary syndrome, highly symptomatic patients, left main stenosis, or left ventricular ejection fraction <35%.
Although the overall interpretation of this trial was neutral, there continues to be much discussion on the design and outcome of the ISCHEMIA study, which cannot be fully reviewed here.
One method to select functionally significant stenoses is with the use of fractional flow reserve (FFR) which we will discuss next.
The FAME studies
Lesions thought to be angiographically/anatomically significant may not necessarily be flow-limiting. FFR involves a pressure wire being passed across the stenosis, and measures the pressure difference across the lesion. The efficacy of FFR measurements in guiding DES placement in patients with multivessel CAD has been shown by earlier data from the FAME (Fractional Flow Reserve Versus Angiography in Multivessel Evaluation) study of 20 medical centres in the US and Europe.34 Researchers randomly assigned 1,005 patients to PCI guided by angiography alone, or by angiography and FFR measurement using a pressure sensor mounted on a guidewire (PressureWire,® Certus). Patients assigned to angiography-guided PCI underwent stenting of all indicated lesions, whereas those assigned to FFR underwent stenting of indicated lesions only if the measurement was 0.80 or less.
The authors found that treating patients, using routine measurement of FFR for guidance, significantly reduced rates of the composite end point of death, non-fatal MI, and repeat revascularisation at one year. Results showed a one-year event rate of 18.3% in the angiography group and 13.2% in the FFR group. At one year, 78% of angiography patients were free from angina compared with 81% of FFR patients.
The FAME trial represented a true ‘paradigm shift’ for decision-making in coronary revascularisation. The weak correlation between angiographically intermediate stenoses and flow limitation during hyperaemic stress had been previously recognised35 and was confirmed in the FAME trial. A subanalysis of the FAME data investigated the relationship between angiographic and functional severity of coronary artery stenosis in multivessel disease. Interestingly, among 509 patients with angiographically defined multivessel disease, only 46% had ‘functional multivessel disease’ (i.e. ≥2 coronary arteries with an FFR ≤0.80).36 Angiography also did not appear accurate in assessing the functional significance of a coronary stenosis in the 70% to 90% diameter stenosis category.36 The FAME and other FFR trials should not necessarily imply that all elective PCI procedures must be done only after functional evaluation of the stenosis.37 Nevertheless, they definitely accredit FFR (figure 5) as a useful tool to help physicians identify ischaemia-producing lesions and avoid unnecessary stenting in selected situations. Five-year follow-up of patients in the FAME study confirmed long-term safety of FFR-guided PCI for multivessel disease. Major adverse cardiac events occurred in 31% of patients in the angiography-guided group versus 28% in the FFR-guided group (p=0.31).38
The benefit of FFR and stenting only haemodynamically significant lesions is underlined by the FAME 2 trial,39 which showed that patients with FFR-guided stenting plus the best available medical therapy had superior outcomes to those treated with medical therapy alone.
For patients found to have a significant stenosis with FFR, the primary end point (a composite of death, MI, or urgent revascularisation) occurred in 4.3% of those in the PCI plus medical therapy group versus 12.7% of those in the medical therapy group. The difference was driven by lower rates of urgent revascularisation in the PCI group than in the medical therapy only group. Five-year follow-up of patients in the FAME 2 trial showed that benefits of an initial FFR-guided PCI strategy were maintained. The rate of the primary end point (composite as described above) was lower in the PCI group than the medical therapy group (13.9% vs. 27.0%; hazard ratio 0.46; 95% CI 0.34–0.63; p<0.001). Notably this was not driven by death nor MI but rather by hospitalisation for chest pain and urgent repeat revascularisation. Patients without haemodynamically significant stenoses had a favourable long-term outcome with only medical therapy.40
Besides multivessel disease, there are other settings of particular interest for evaluation of a functional lesion, such as equivocal left main stenosis,41 which often guides the choice between percutaneous and surgical revascularisation, and non-culprit lesions in patients with acute MI.42
Currently the FAME 3 trial, a mulicentre randomised controlled non-inferiority trial comparing FFR-guided PCI with CABG is being performed to investigate a primary end point of major adverse cardiac events at one year.43
DEFINE-FLAIR and iFR-SWEDEHEART studies
Instantaneous wave-free ratio (iFR) is an alternative method of measuring the haemodynamic significance of coronary lesions and is a method that does not require the administration of adenosine. During assessment of stenoses with FFR, adenosine, a potent vasodilator, must be given to induce maximal hyperaemia. The use of adenosine is not without additional risks and costs, and avoiding its use is therefore preferable. The DEFINE-FLAIR (Functional Lesion Assessment of Intermediate Stenosis to Guide Revascularisation) trial (n=2,492) investigated iFR- compared to FFR-guided coronary revascularisation in patients with CAD and found non-inferiority in the primary end point (one-year risk of major adverse cardiac events – composite of all cause mortality, nonfatal MI, or unplanned revascularisation).44 The primary end point occurred in 78 of 1,148 patients (6.8%) in the iFR group, compared to 83 of 1,182 patients (7.0%) in the FFR group (difference in risk, -0.2 percentage points; 95% CI, -2.3 to 1.8; p<0.001 for non-inferiority). In addition, the use of iFR was associated with fewer adverse procedural signs and symptoms and a shorter procedural time.
Similarly, non-inferiority was demonstrated in the iFR-SWEDEHEART (The Instantaneous Wave-free Ratio versus Fractional Flow Reserve in Patients with Stable Angina Pectoris or Acute Coronary Syndrome) trial (n=2,037), a multi-centre randomised controlled open-label clinical trial that investigated the same primary end point in patients with CAD or ACS.45 Recent meta-analyses have confirmed the improvements seen with FFR-guided revascularisation and shown no difference in outcomes between iFR and FFR.46–8
There are also a number of new modalities to consider. For further information, see the following references.
- – pressure-wired based modalities: non-hyperaemic pressure ratio and resting full-cycle ratio (RFR);49,50 diastolic pressure ratio51
- – angiography-based modalities: quantitative flow ratio (QFR)52
- – optical coherence tomography (OCT)-derived modalities: OCT-based FFR (OFR)53
The contribution from coronary stents
The use of DES has substantially improved clinical outcomes in patients undergoing PCI and technological advances with second-generation DES (e.g. zotarolimus, everolimus or ridaforolimus-eluting stents; improvements in stent design such as thinner struts, better polymers and improved deliverability). These have reduced, to some extent, the drawbacks of first-generation DES (e.g. sirolimus and paclitaxel stents).1 Outcomes are similar between the current second-generation DES, however, and there is not much to differentiate them – see recent Onyx ONE registry.54
Initially, bare-metal stents (BMS) were used in PCI but were associated with high rates of stent restenosis, which limited their long-term efficacy. DES, which released anti-proliferative drugs over a number of months, reduced the rate of restenosis but early generation DES were associated with higher rates of stent thrombosis given reduced endothelialisation. Newer-generation DES, however, have lower rates of stent thrombosis than BMS.55 A recent individual patient data meta-analysis, gathering individual data for 26,616 patients from 20 randomised trials, showed the risk of the primary outcome (composite of cardiac death or MI) was significantly reduced with DES compared with BMS, as was risk of stent thrombosis and target-vessel revascularisation. In particular though, the benefit of DES over BMS in the primary outcome was only observed up to one year.
Second-generation DES, compared to first-generation DES and BMS, have been shown to reduce rates of revascularisation by 39-61% and have improved cardiovascular outcomes, including death, MI and stent thrombosis.56 Consequently, first-generation stents are no longer routinely used.
Between second-generation DES, a number of large registries have shown equivalence of multiple stent platforms with everolimus-eluting stents (EES). However, a meta-analysis by Bangalore et al. suggests that EES may have the lowest target-vessel revascularisation rate and the greatest reduction in MI and stent thrombosis.57 Importantly, this meta-analysis also showed that, with any DES, there was no increase in the risk of long-term safety outcomes, including stent thrombosis, compared with BMS. Similarly, a pooled analysis of three prospective registries of various second-generation DES for left main disease observed similar results between stent types in real-world clinical practice. For the primary outcome of three-year target-vessel failure, there was no significant difference between second-generation DES (e.g. cobalt-chromium EES, biodegradable polymer biolimus-eluting stent [BP-BES], platinum-chromium EES [PtCr-EES), resolute zotarolimus-eluting stent) except for a higher risk of primary outcome with PtCr-EES compared to BP-BES.58 Strut thickness has also been shown to affect outcomes. A meta-analysis of 10 trials (n=11,658), which evaluated newer-generation ultrathin strut DES compared to thicker strut second-generation DES, showed the former to be associated with a 16% reduction in target lesion failure at one year (relative risk 0.84; 95% CI, 0.72–0.99) predominantly due to fewer MIs.59
However “the quest for the optimal coronary stent continues…” (see figure 6)60 and third-generation stents, using newer technology (e.g. biodegradable polymers, polymer-free stents, biodegradable stents) are being investigated.61,62
Much interest has focused on the bioabsorbable or biodegradable polymer scaffolds that were designed to overcome limitations of metallic DES63 It was thought they could offer a number of advantages, such as providing a temporary rather than a permanent scaffold so as to preserve normal coronary vasomotion, ability to undertake future bypass grafting and result in less long-term foreign-body responses that represent an on-going risk of restenosis or stent thrombosis.64 Whilst the Absorb Bioresorbable Vascular Scaffold was the first Food and Drug Administration-approved device, long-term results have shown increased rates of stent thrombosis, target vessel infarct and target-lesion revascularisation, leading to withdrawal from the market.65,66
Next-generation bioresorbable scaffolds will need to address these concerns.64 How well a stent is deployed at the site of stenosis is important and studies have looked at factors e.g. appropriate stent sizing, degree of stent expansion, and post-procedure lumen size. To best do its job, a stent must be delivered optimally and how well it is delivered affects cardiovascular outcomes. Intracoronary imaging, e.g. intravascular ultrasound (IVUS) or OCT, are methods that can help improve stent sizing, delivery, identify mechanisms of stent failure, and help reduce rates of major cardiovascular events.67
Discussion of methods of intracoronary imaging is outside the scope of this e-learning module. For further information, see references 68 and 69.
For reviews on intracoronary imaging, see references 70-2.
Chronic total occlusion
Chronic total occlusions (CTO) are identified in 15–30% of all patients referred for coronary angiography. A worse prognosis has been attached to CTO. Revascularisation needs to be discussed in patients with symptoms of occlusion or large ischaemic areas. PCI of CTO is technically challenging and requires familiarity with advanced techniques and specialised equipment, although improvements in catheter and wire technology, and greater operator experience have translated to improved success rates. Surgical treatment, with the implantation of a distal bypass graft, is also a valid option for discussion.73
The EUROCTO (Randomized Multicentre Trial to Compare Revascularization with Optimal Medical Therapy for the Treatment of Chronic Total Occlusions) trial has shown symptomatic improvement with PCI for CTO with improvements in angina frequency and quality of life.74 A systematic review of 25 observational studies has demonstrated improved outcomes in survival, angina burden and requirement for CABG, compared to patients who failed revascularisation.75 However, recent randomised studies have failed to demonstrate a difference in the incidence of major adverse cardiovascular events and further research is needed. Nevertheless, important factors when performing CTO-PCI include not only the need to seek demonstrable objective evidence of myocardial viability in the CTO territory but also the risks of greater contrast volume, greater fluoroscopy time, and risks of complications.
Coronary artery bypass graft surgery (CABG) versus other interventions
The many studies comparing these two revascularisation strategies have shown that neither PCI nor CABG alone can provide a solution for the entire spectrum of stable angina patients who need revascularisation.1 However, CABG results in more complete revascularisation than PCI, and the placement of bypass grafts on the mid-coronary vessel makes the complexity of proximal lesions less relevant for the procedure, especially when there are chronic proximal occlusions.
As described above, patients with left main stem, one- or two-vessel CAD with proximal stenosis of the left anterior descending artery (LAD), or triple-vessel CAD, particularly in patients with diabetes, should be considered for CABG, providing there is no contra-indication (See ESC/EACTS guidelines – bottom table page 1071). A number of studies have compared multivessel PCI with DES versus CABG. These have indicated equivalent mortality outcomes from both procedures, but superior symptom resolution and reduced risk of a repeat revascularisation procedure with CABG.76
The SYNTAX (Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) trial (n=1,800) investigated PCI (using first-generation paclitaxel-eluting stents) against CABG for previously untreated triple-vessel or left main CAD (or both).76 This landmark trial studied a non-inferiority primary end point of major adverse cardiac or cerebrovascular events during 12 months follow-up after randomisation and found a significantly higher rate of major adverse events with PCI compared to CABG (17.8% vs. 12.4%; p=0.002) driven primarily by an increased rate of repeat revascularisation. Subgroup analyses showed a significant advantage of CABG especially in patients with complex coronary disease (intermediate/high SYNTAX scores for three-vessel disease or high tercile for left main disease).78 Five-year follow-up showed a significantly higher rate of major adverse events with PCI compared to CABG (37.5% vs. 24.2%, p<0.001).79 On the basis of the available data, CABG is the preferred revascularisation option for these patients, but PCI remains a very reasonable option for all other patients, for patients who choose not to undergo surgery, or in whom CABG is either contraindicated or considered high risk.
Further evidence on the optimal revascularisation strategy for left main CAD has been suggested by the EXCEL (Evaluation of XIENCE versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization) trial. This was an open-label multicentre randomised trial comparing everolimus-eluting stents to CABG for treatment of left main CAD.80 It assigned 1,905 patients with low or intermediate SYNTAX scores (a score of anatomic complexity and extent of CAD) and at three years’ follow-up, the non-inferiority primary end point (rate of composite of all-cause mortality, stroke, or MI at three years) was 15.4% for PCI compared to 14.7% with CABG (p=0.02 for non-inferiority; hazard ratio 1.00; 95% confidence interval 0.79-1.26; p=0.98 for superiority). A recent analysis (n=1,840) of four-year outcomes of the EXCEL trial based on SYNTAX score showed a mean score of 26.5±9.3 and four-year rates of the primary end point were similar between PCI and CABG (18.6% vs. 16.7%; p=0.40). This did not vary according to SYNTAX score, although the relative and absolute hazard of major adverse events with PCI compared with CABG rose progressively with SYNTAX score.81 The recently reported five-year outcomes of the EXCEL trial have shown no significant difference between PCI and CABG.82 However as we go to press, these results are the subject of much controversy.
In general, the choice of revascularisation strategy must integrate angiographic and clinical findings, consider co-morbidities, and weigh the benefits and risks of each intervention. Scoring systems evaluating anatomic complexity of PCI (SYNTAX I/II scores)77 or surgical risk (EuroSCORE II)83,84 may be useful to guide treatment but clinical judgement cannot be replaced by any single or combined scoring system. Table 2 shows a comparison of two revascularisation strategies (PCI and CABG) for stable angina patients.
Table 2. A comparison of revascularisation strategies for stable angina patients
| Favours PCI | Favours CABG |
|---|---|
Clinical characteristics:
|
Clinical characteristics:
|
Anatomical and technical aspects:
|
Anatomical and technical aspects:
|
Need for concomitant interventions:
|
|
| Key: CABG = coronary artery bypass graft; EF = ejection fraction; LV = left ventricular; PCI = percutaneous coronary intervention | |
Current European and US guidelines agree about the necessity to discuss the revascularisation strategy for patients with unprotected left main or complex CAD within a multidisciplinary team (“Heart Team approach”).1,3 Observational data suggest that implementation of this recommendation is very important. A Canadian study, for example, found a huge variation in the ratio of PCI to CABG across different centres.86 Most of the variation was among patients with complex disease, and the physician performing the angiogram appeared to be the key determinant of subsequent strategy of treatment.85
The SYNTAX III Revolution trial offers further decision-making by the multi-disciplinary heart team and tested the hypothesis that their treatment recommendation based on non-invasive CTCA assessment agreed with that derived from conventional angiography, in patients with left main or triple-vessel CAD.86 It was an international multi-centre trial (n=223) that randomised separate heart teams to provide a treatment recommendation of CABG, PCI or equipoise between the two, and had a primary end point of agreement between heart teams on revascularisation strategy. CABG was recommended in 28% of cases with CTCA; 26% with conventional angiography. Agreement of treatment decisions based on CTCA and conventional angiography was high and there was agreement on the coronary segments to be revascularised in 80% of cases.
Coronary artery bypass graft surgery
This module has focused on PCI as the predominant form of revascularisation. Clearly there are indications for CABG surgery. While we will not address this extensively in this module, readers are advised to refer to the recent European guidelines on myocardial revascularisation.1 Such surgery, using venous or arterial conduits, is highly effective in relieving angina.
CABG has also been shown to improve prognosis in patients with left main stem, three-vessel disease (with or without left ventricular systolic dysfunction), or two vessel disease involving the proximal left-anterior descending artery. CABG does not reduce the risk of myocardial infarction. A single graft to one artery may improve symptoms, but has no prognostic benefit.
Conclusion
While both PCI and CABG have witnessed significant technological advances, in particular the use of DES in PCI and of arterial grafts in CABG, their role in the treatment of patients presenting with stable CAD is being challenged by advances in medical treatment, referred to as optimal medical therapy (OMT), which include intensive lifestyle and pharmacological management.1
Formulation of the best possible revascularisation approach in selected patients, taking into consideration the social and cultural context also, will require interaction between cardiologists and cardiac surgeons, referring physicians or other specialists as desirable. Multi-disciplinary team meetings (MDTs) are designed for this purpose. Patients also will need help in taking informed decisions about their treatment, and the most valuable advice will likely be provided to them by the Heart Team.1
The following clinical cases are not part of the testing for this module but may be useful background information to illustrate revascularisation.
How and when I use a coronary pressure guidewire
by Dr Francesco Saia, University of Bologna, Italy

In common practice, many patients with known CAD and/or an intermediate or high pre-test likelihood of ischaemia are catheterised without prior functional testing.87,88 In these cases, the measurement of fractional flow reserve (FFR)* might be very helpful and is recommended from current guidelines for detection of ischaemia-producing lesions.1,3
The most common applications of FFR are the following:
- Intermediate stenosis (50% to 70% diameter stenosis)
- Stenosis 70% to 90% without evidence of ischaemia
- Equivocal left main stenosis
- Equivocal aorto-ostial lesions
- Side-branch lesions
- Multi-vessel disease when the functional effect of individual lesions is uncertain
- Intermediate in-stent restenosis lesions.
In fact, despite the demonstration that angiographic estimation of a stenosis presents only a weak correlation with its functional significance,35 most severe stenoses (i.e. >90%) almost invariably produce an FFR <0.80 and can be treated without testing in most instances (figure 7). Similarly, in the presence of typical angina not relieved by medical therapy, there is a good rationale in treating isolated stenoses 70 to 90% even in the absence of a stress test, provided other causes of chest pain have been reasonably excluded. In these situations, FFR might anyway further support invasive treatment.
*Fractional flow reserve (FFR) is an index of the physiological significance of a coronary stenosis, defined as the ratio of maximal blood flow in a stenotic artery to normal maximal flow. FFR in a normal coronary artery equals 1.0. An FFR value of 0.80 or less identifies ischaemia-causing coronary stenoses with an accuracy of more than 90%.
Clinical case 1
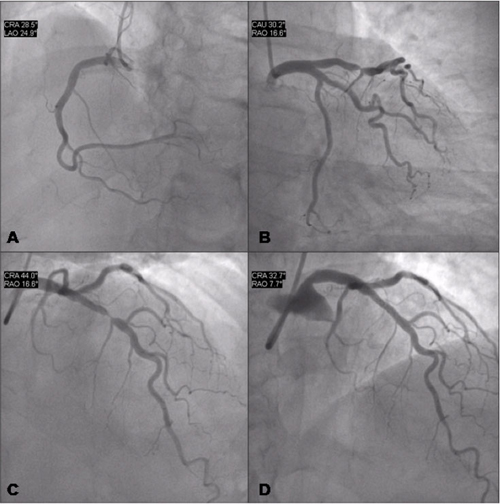
Figure 7 shows the coronary angiography of a 52-year-old woman presenting stable angina symptoms for three months (Canadian Cardiovascular Society (CCS) class 3). Administration of appropriate medical treatment (aspirin, full dose beta blockers, nitrates and simvastatin) reduced but did not resolve symptoms. A treadmill test showed severe ischaemia after three minutes of exercise using the modified Bruce protocol.
The right coronary artery (Figure 7A) and the left circumflex artery (Figure 7B) were free of significant disease. The left anterior descending coronary artery showed a tight (<90%) stenosis at the mid portion (Figure 7C).The patient had a very active lifestyle and, after informed consent, she was treated with coronary angioplasty and stenting with a good angiographic result (Figure 7D).
Practice point
In this patient, the combination of angina pectoris despite therapy, her willingness to resolve her angina without further medication, with the documentation of a tight coronary stenosis and previous documentation of myocardial ischaemia, clearly make functional lesion testing unnecessary and decision making very straightforward.
Single photon emission computed tomography (SPECT) is often used to confirm ischaemia in patients with stable angina. The findings of angiography might not always be completely consistent with the area of ischaemia found by myocardial perfusion imaging. In such cases, FFR is an extremely valuable tool to select lesions warranting treatment, with solid evidence provided by several trials (figures 8 and 9).
Clinical case 2
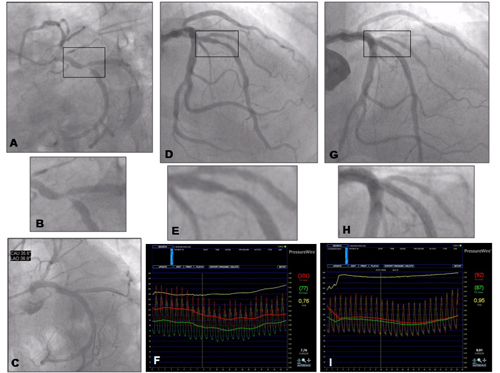
Coronary angiography of a 68-year-old man with a clinical history of high cholesterol and hypertension, who had suffered an MI 18 years previously and was treated with stropetokinase IV. He had remained asymptomatic since. Three years before admission, there was documentation of a positive stress test without angina with a high threshold of ischaemia, which had been managed conservatively. In the previous six months, the patient presented effort angina (CCS class 3). Myocardial scintigraphy showed a previous inferior wall necrosis with mild peri-lesional reversible ischaemia. Due to the persistence of symptoms despite full medical treatment, the patient underwent coronary angiography. The right coronary artery was hypoplasic. There was a tight stenosis (>90%) of the dominant left circumflex artery (figure 8 A and B), which was consistent with the SPECT findings and so was treated with angioplasty and stenting (figure 8 C shows the final result post-stenting). There was an intermediate stenosis of the LAD (figure 8 D and E, stenosis diameter at quantitative coronary analysis was 58%), whereas there was no evidence of ischaemia with scintigraphy in the LAD territory. This was an ideal case for functional testing. Measurement of FFR was 0.76 (figure 8 F) and so this lesion was also stented. Figure 8 G and H show the final result after stenting, with normalisation of FFR shown in figure 8 I.
As previously mentioned, there are other interesting practical applications of FFR. Of particular interest is evaluation of equivocal left main stenosis,41 where FFR is probably more reliable than the widely used intravascular ultrasound and can be used either as an alternative or as a complementary methodology to test such lesions.
Clinical case 3
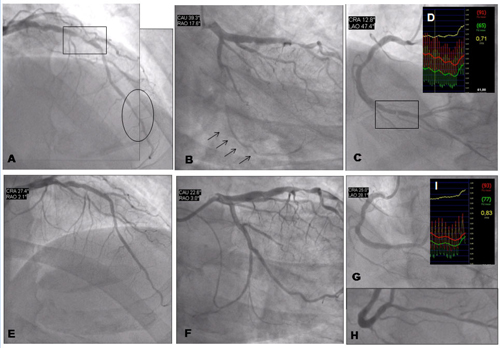
Coronary angiography of a 69-year-old man with multiple risk factors (type 2 insulin-requiring diabetes mellitus, high cholesterol, hypertension, family history), chronic occlusion of the left internal carotid artery and previous surgery for significant stenosis of the right internal carotid, who was virtually asymptomatic (mild effort dyspnoea). SPECT imaging was prescribed, which showed severe reversible anterior, apical and lateral ischaemia. The subsequent coronary angiography demonstrated severe three-vessel disease with a double stenosis of the LAD at the mid and distal portions (figure 9A), chronic total occlusion of the left circumflex artery (figure 9B), which was visible through collateral flow (arrows). There was also a tight stenosis of the distal right coronary artery (figure 9C), also presenting intermediate stenosis of the posterior descending artery (figure 9H). The patient’s case was discussed by the local Heart Team. Echocardiography demonstrated a severe hypokinesia of the inferior wall, but left ventricular ejection fraction was normal. Renal function was mildly impaired (GFR 56 ml/min/m2), SYNTAX score was 13.5 (low risk), log EuroSCORE was 2.1%. It was considered that the LAD at the site of eventual grafting was not optimal (presence of a distal stenosis) and the risk of cerebrovascular events was moderately high; on the other hand, the interventional cardiologist predicted a high likelihood of achieving a complete revascularisation. Based on these data, multi-vessel PCI was planned. The operator did not consider FFR for the LAD and the left circumflex artery (as there was clear documentation of ischaemia with the SPECT). Treatment of the right coronary artery was also planned upfront with the aim of achieving a complete revascularisation given the severity of the stenosis (>90%). Nevertheless, FFR was used to confirm the ischaemic significance of the stenosis (there was suspicion of previous MI with echocardiography, which was unconclusive on ECG). FFR was 0.71 (upper right box). The result of PCI after multiple drug-eluting stent implantation is showed by figure 9 E, F and G. FFR post-procedure of the RCA was 0.83 (box), which was considered optimal and caused by the diffuse disease of posterior descending artery.
Another extremely interesting field of application seems the evaluation of non-culprit lesions in patients with acute coronary syndromes and ST-elevation myocardial infarction in particular. A recent study during the acute phase of acute coronary syndromes has, in fact, demonstrated that FFR can reliably assess severity of non-culprit coronary artery stenoses in this setting.42 This may ease the decision about the need for additional revascularisation and contribute to a better risk stratification (figure 10).
Clinical case 4
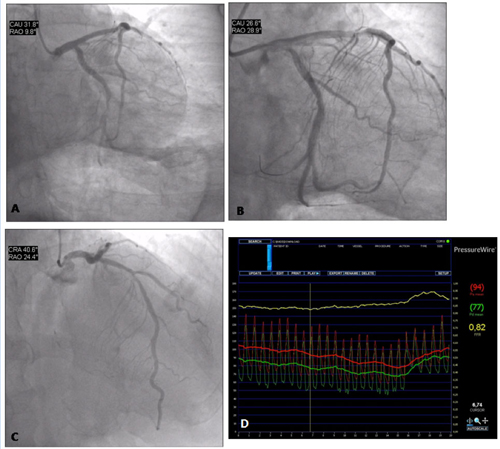
Coronary angiography of a 63-year-old man with infero-posterior ST-elevation MI. The patient was a smoker and did not present a clinical history of angina. The culprit lesion was the thrombotic occlusion of the dominant left circumflex artery (figure 10 A), which was successfully treated with primary angioplasty and stenting (figure 10 B). There was a 50-60% stenosis of the proximal LAD (figure 10 C). The functional severity of the stenosis was assessed during the same procedure with FFR, which was found to be 0.82 (figure 10 D). On the basis of this finding, the patient was discharged home without further risk stratification. Six months later he was asymptomatic and with a negative exercise test (treadmill test).
close window and return to take test
References
- Neumann F-J, Sousa-Uva M, Ahlsson A, et al. ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. https://dx.doi.org/10.1093/eurheartj/ehy394
- National Institute for Health and Care Excellence (NICE). Stable angina: Methods, evidence and guidance (July 2011). National Clinical Guidelines Centre 2011. https://www.nice.org.uk/guidance/cg126
- Levine GN, Bates ER, Blankenship JC et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011;58:e44–e122. http://dx.doi.org/10.1016/j.jacc.2011.08.007
- Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. J Am Coll Cardiol 2012;60:e44–e164. https://dx.doi.org/10.1016/j.jacc.2012.07.013
- Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease. J Am Coll Cardiol. 2014;64:1929–49. https://dx.doi.org//10.1016/j.jacc.2014.07.017
- Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery. J Am Coll Cardiol 2011;58:e123–e210. https://dx.doi.org/10.1016/j.jacc.2011.08.009
- Patel MR, Calhoon JH, Dehmer GJ et al. Coronary revascularisation writing group. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease. J Am Coll Cardiol 2017;69:2212–41. https://dx.doi.org/10.1016/j.jacc.2017.02.001
- OECD. Health at a glance: Europe 2010. OECD iPublishing, 2010. http://dx.doi.org/10.1787/health_glance-2010-en
- Bangalore S, Gupta N, Généreux P, Guo Y, Pancholy S, Feit F. Trend in percutaneous coronary intervention volume following the COURAGE and BARI-2D trials: onsight from over 8.1 mililion percutaneous coronary interventions. Int J Cardiol 2015;183:6–10. https://dx.doi.org/10.1016/j.ijcard.2015.01.053
- Carpenter JR, Mansfield R. Management of chronic, stable, ischaemic heart disease. In Purcell H , Kalra PR (eds.) Cardiology. Elsevier Mosby, 2005.
- Stone GW, Kappetein P, Sabik JF, et al. for the EXCEL Trial Investigators. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med 2019 (published online September 20th 2019). https://dx.doi.org/10.1056/NEJMoa1909406
- Choo WK, Amersey R. The joint cardiology-cardiothoracic multidisciplinary team (MDT) meeting: patient characteristics and revascularisation outcomes. Br J Cardiol 2009;16:292-4. https://bjcardio.co.uk/2009/11/the-joint-cardiology-cardiothoracic-multi-disciplinary-team-mdt-meeting-patient-characteristics-and-revascularisation-outcomes/ (last accessed 4th November 2019)
- Ludman PF on behalf of the British Cardiovascular Intervention Society. BCIS audit returns: adult interventional procedures (1st April 2017–31st March 2018).
http://www.bcis.org.uk/wp-content/uploads/2019/01/BCIS-Audit-2017-18-data-for-web-ALL-excl-TAVI-as-22-01-2019.pdf - OECD/EU. Health at a Glance: Europe 2016 – State of Health in the EU Cycle. Paris: OECD Publishing, 2016. https://ec.europa.eu/health/sites/health/files/state/docs/health_glance_2016_rep_en.pdf
- Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary revascularization trends in the United States, 2001–2008. JAMA 2011;305:1769–76. http://dx.doi.org/10.1001/jama.2011.551
- Alexander JH, Smith PK. Coronary-artery bypass grafting. N Engl J Med 2016;374:1954–64. https://dx.doi.org/10.1056/NEJMra1406944
- Shaw LJ, Berman DS, Maron DJ et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008;117:1283–91. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.743963
- Boden WE, O’Rourke RA, Teo KK et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–16. http://dx.doi.org/10.1056/NEJMoa070829
- Muller O, Mangiacapra F, Ntalianis A et al. Long-term follow-up after fractional flow reserve-guided treatment strategy in patients with an isolated proximal left anterior descending coronary artery stenosis. JACC Cardiovasc Interv 2011;4:1175–82. http://dx.doi.org/10.1016/j.jcin.2011.09.007
- Bech GJ, De Bruyne B, Pijls NH et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation 2001;103:2928–34.
- Erne P, Schoenenberger AW, Burckhardt D et al. Effects of percutaneous coronary interventions in silent ischemia after myocardial infarction: the SWISSI II randomized controlled trial. JAMA 2007;297:1985–91. http://dx.doi.org/10.1001/jama.297.18.1985
- Simoons ML, Windecker S. Controversies in cardiovascular medicine: Chronic stable coronary artery disease: drugs vs. revascularization. Eur Heart J 2010;31:530–41. http://dx.doi.org/10.1093/eurheartj/ehp605
- Hochman JS, Lamas GA, Buller CE et al. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med 2006;355:2395–407. http://dx.doi.org/10.1056/NEJMoa066139
- Pitt B, Waters D, Brown WV et al. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. Atorvastatin versus Revascularization Treatment Investigators. N Engl J Med 1999;341:70–6. http://dx.doi.org/10.1056/NEJM199907083410202
- Windecker S, Stortecky S, Stefanini GG, et al. Revascularisation versus medical treatment in patients with stable coronary artery disease: network meta-analysis. BMJ 2014;348:g3859. https://dx.doi.org/10.1136/bmj.g3859
- Al-Lamee R, Howard JP, Sun-Shin MJ, et al. Instantaneous wave-free ratio as predictors of the placebo-controlled response to percutaneous coronary intervention in stable single-vessel coronary artery disease. Circulation 2018;138:1780–92. https://dx.doi.org/10.1161/CIRCULATIONAHA.118.033801
- Kumbhani DJ. Objective randomised blinded investigation with optimal medical therapy of angioplasty in stable angina – ORBITA. American College of Cardiology latest in cardiology. https://www.acc.org/latest-in-cardiology/clinical-trials/2017/11/02/08/26/orbita (last accessed 4th November 2019).
- Controversies in interventional cardiology I: interventional management in stable coronary artery disease. Presented at: SCAI 2018, April 25th, 2018. San Diego, CA., USA.
- Frye RL, August P, Brooks MM et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360:2503–15. http://dx.doi.org/10.1056/NEJMoa0805796
- Farkouh ME, Domanski M, Sleeper LA, et al. for the FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012;367:2375–84. https://dx.doi.org/10.1056/NEJMoa1211585
- Borden WB, Redberg RF, Mushlin AI, Dai D, Kaltenbach LA, Spertus JA. Patterns and intensity of medical therapy in patients undergoing percutaneous coronary intervention. JAMA 2011;305:1882–9. http://dx.doi.org/10.1001/jama.2011.601
- Ahmed B, Dauerman HL, Piper WD, et al. Recent changes in practice of elective percutaneous coronary intervention for stable angina. Circ Cardiovasc Qual Outcomes 2011;4:300–5. http://dx.doi.org/10.1161/CIRCOUTCOMES.110.957175
- Hochman JS. Presentation of the ISCHEMIA trial results. Late-breaking session at the American Heart Association Annual Scientific Sessions (AHA 2019), Philadelphia, PA, USA. 16th November 2019. https://www.ischemiatrial.org/ischemia-study-results
- Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213–24. http://dx.doi.org/10.1056/NEJMoa0807611
- Pijls NH, De Bruyne B, Peels K, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med 1996;334:1703–8. http://dx.doi.org/10.1056/NEJM199606273342604
- Tonino PA, Fearon WF, De Bruyne B, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol 2010;55:2816–21. http://dx.doi.org/10.1016/j.jacc.2009.11.096
- Ellis SG. Refining the art and science of coronary stenting. N Engl J Med 2009;360:292–4. http://dx.doi.org/10.1056/NEJMe0809422
- van Nunen LX, Zimmermann FM, Tonino PA, et al. Fractional flow reserve versus angiography for the guidance of PCI in patients with multivessel coronary artery disease (FAME): 5-year follow-up of a randomised controlled trial. Lancet 2015;386:1853–60. https://dx.doi.org/10.1016/S0140-6736(15)00057-4
- De Bruyne B, Pijls NH, Kalesan B et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 2012:367:991-1001. https://dx.doi.org/10.1056/NEJMoa1205361
- Xaplanteris P, Fournier S, Pijls NHJ, et al. Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med 2018;379:250–9. https://dx.doi.org/10.1056/NEJMoa1803538
- Hamilos M, Muller O, Cuisset T et al. Long-term clinical outcome after fractional flow reserve-guided treatment in patients with angiographically equivocal left main coronary artery stenosis. Circulation 2009;120:1505–12. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.850073
- Ntalianis A, Sels JW, Davidavicius G et al. Fractional flow reserve for the assessment of nonculprit coronary artery stenoses in patients with acute myocardial infarction. JACC Cardiovasc Interv 2010;3:1274–81. http://dx.doi.org/10.1016/j.jcin.2010.08.025
- A Comparison of Fractional Flow Reserve-Guided Percutaneous Coronary Intervention and Coronary Artery Bypass Graft Surgery in Patients With Multivessel Coronary Artery Disease (FAME 3). ClinicalTrials.gov Identifier: NCT02100722 https://clinicaltrials.gov/ct2/show/NCT02100722 (last accessed 5th November 2019)
- Davies JE, Sen S, Dehbi H-M, et al. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. N Engl J Med 2017;376:1824–34 https://www.nejm.org/doi/full/10.1056/NEJMoa1700445
- Götberg M, Christiansen EH, Ingibjörg IJ, et al. for the iFR-SWEDEHEART Investigators. Instantaneous wave-free ratio versus fractional flow reserve to guide PCI. N Engl J Med 2017;376:1813–23. https://dx.doi.org/10.1056/NEJMoa1616540
- De Rosa S, Polimeni A, Petraco R, Davies JE, Indolfi C. Diagnostic performance of the instantaneous wave-free ratio: comparison with fractional flow reserve. Circ Cardiovasc Interv 2018;e004613. https://dx.doi.org/10.1161/CIRCINTERVENTIONS.116.004613
- Zimmerman FM, Omerovic E, Fournier S, et al. Fractional flow reserve-guided percutaneous coronary interventions vs. medical therapy for patients with stable coronary lesions: meta-analysis of individual patient date. Eur Heart J 2019;40:180–6. https://dx.doi.org/10.1093/eurheartj/ehy812
- Verardi R, Fioravanti F, Barbero U, et al. Network meta-analysis comparing iFR versus FFR versus coronary angiography to drive coronary revascularization. J Interv Cardiol 2018;31:725–30. https://dx.doi.org/10.1111/joic.12551
- Svanerud J, Ahn JM, Jeremias A, et al. Validation of a novel non-hyperaemic index of coronary artery stenosis severity: the Resting Full-cycle Ratio (VALIDATE RFR) study. Eurointervention 2018;14:806-814. https://doi.org/10.4244/EIJ-D-18-00342
- Aoun J, Lahsaei S, Zahm C, et al. Validation and comparison of non-hyperemic pressure reserve to fractional flow reserve for assessment of coronary artery stenosis: A real world study. Catheter Cardiovasc Interv 2019;93:250–5. https://doi.org/10.1002/ccd.27834
- Ligthart J, Masdjedi K, Witberg K, et al. Validation of resting diastolic pressure ratio calculated by a novel algorithm and its correlation with distal coronary artery pressure to aortic pressure, instantaneous wave-free ratio, and fractional flow reserve. Circ Cardiovasc Interv 2018;11:e006911. https://doi.org/10.1161/CIRCINTERVENTIONS.118.006911
- Xu B, Tu S, Qiao S, et al. Diagnostic accuracy of angiography-based quantitative flow ratio measurements for online assessment of coronary stenosis. J Am Coll Cardiol. 2017;70:3077–87. https://doi.org/10.1016/j.jacc.2017.10.035
- Yu W, Huang J, Jia D, et al. Diagnostic accuracy of intracoronary optical coherence tomography-derived fractional flow reserve for assessment of coronary stenosis severity. Eurointervention. 2019;15:189-197. https://doi.org/10.4244/EIJ-D-19-00182
- TCT The heartbeat. News from TCT2019. https://www.tctmd.com/news/onyx-one-shorter-dapt-plus-zes-noninferior-polymer-free-stent-high-bleeding-risk-patients (last accessed 5th November 2019)
- Piccolo R, Bonaa KH, Efthimiou O, et al. Drug-eluting or bare-metal stents for percutaneous coronary intervention: a systematic review and individual patient data mata-analysis of randomised controlled trials. Lancet 2019;393:2503-10. https://dx.doi.org/10.1016/S0140-6736(19)30474-X
- Bangalore S, Kumar S, Fusaro M, et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed treatment comparison analysis of 117,762 patient-years of follow-up from randomized trials. Circulation 2012;125:2873-91. https://dx.doi.org/10.1161/CIRCULATIONAHA.112.097014
- Bangalore S, Toklu B, Amoroso N, et al. Bare-metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. BMJ 2013;347:f6625. https://dx.doi.org/10.1136/bmj.f6625
- Lee PH, Kwon O, Ahn JM, et al. Safety and effectiveness of second-generation drug-eluting stents in patients with left main coronary artery disease. J Am Coll Cardiol 2018;71:832-41. https://dx.doi.org/10.1016/j.jacc.2017.12.032
- Bangalore S, Toklu B, Patel N, Feit F, Stone GW. Newer-generation ultrathin strut drug-eluting stents versus older second-generation thicker strut drug-eluting stents for coronary artery disease. Circulation 2018;138:2216-26. https://dx.doi.org/10.1161/CIRCULATIONAHA.118.034456
- Wilson WM, Cruden NLM. Advances in coronary stent technology:current expectations and new developments. Research Reports in Clinical Cardiology. 2013;4:85–96. http://www.dovepress.com/advances-in-coronary-stent-technology-current-expectations-and-new-dev-peer-reviewed-article-RRCC
- Akin I,Schneider H, Ince H, et al. Second-and third-generation drug-eluting coronary stents: progress and safety. Herz 2011;36:190-6. https://dx.doi.org/10.1007/s00059-011-3458-z
- Lee D-H, de la Torre Hernandiz JM. The newest generation of drug-eluting stents and beyond. Eur Cardiol Rev 2018;13:54-9. https://dx.doi.org/10.15420/ecr.2018:8:2
- Serruys PW, Chevalier B, Dudek D, et al. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet 2014;385:43–54. http://dx.doi.org/10.1016/S0140-6736(14)61455-0
- Jinnouchi H, Torii S, Sakamoto A, et al. Fully bioresorbable vascular scaffolds: lessons learned and future directions. Nat Rev Cardiol 2019;16:286-304. https://dx.doi.org/10.1038/s41569-018-0124-7
- Serruys PW, Chevalier B, Sotomi Y, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluing metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet 2016;388;2479-91. https://dx.doi.org/10.1016/S0140-6736(16)32050-5
- Buccheri S, Capodanno D. Bioabsorbable stents: only bad news? Eur Heart J Suppl 2019;21(supplB):B28-B30. https://doi.org/10.1093/eurheartj/suz009
- Zhang J, Gao X, Kan J, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation. The ULTIMATE trial. J Am Coll Cardiol 2018;72(24). https://dx.doi.org/10.1016/j.jacc.2018.09.013
- Murray SW. Contemporary coronary imaging from patient to plaque part 1: IVUS-derived virtual histology. Br J Cardiol 2010;17:129-32. https://bjcardio.co.uk/2010/05/contemporary-coronary-imaging-from-patient-to-plaque-part-1-ivus-derived-virtual-histology/ (last accessed 5th November 2019)
- Rathore S, Murray SW, Stables RH et al. From patient to plaque. Contemporary coronary imaging – part 2: optical coherence tomography. Br J Cardiol 2010;17:190-3. https://bjcardio.co.uk/2010/07/from-patient-to-plaque-contemporary-coronary-imaging-part-2-optical-coherence-tomography/ (last accessed 5th November 2019)
- Giavarini A, Kilic ID, Redondo Diéguez A et al. Intracoronary imaging. Heart 2017;103:708–25. https://doi.org/10.1136/heartjnl-2015-307888
- Mintz GS, Guagliumi G. Intravascular imaging in coronary artery disease. Lancet 2017;390:793–809. https://doi.org/10.1016/S0140-6736(17)31957-8
- Scalone G, Niccoli G, Gomez Monterrosas O et al. Intracoronary imaging to guide percutaneous coronary intervention: Clinical implications. Int J Cardiol 2019;274:394–401. https://doi.org/10.1016/j.ijcard.2018.09.017
- Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease. Eur Heart J 2013;34:2949–3003. http://dx.doi.org/10.1093/eurheartj/eht296
- Werner GS, Martin-Yuste V, Hildick-Smith D, et al; EUROCTO trial investigators. A randomized multicentre trial to compare revascularization with optimal medical therapy for the treatment of chronic total coronary occlusions. Eur Heart J 2018 (published online ahead of print 2nd May 2018). https://dx.doi.org/10.1093/eurheartj/ehy220
- Christakopoulos GE, Christopoulos G, Carlino M, et al. Meta-analysis of clinical outcomes of patients who underwent percutaneous coronary interventions for chronic total occlusions. Am J Cardiol 2015;115:1367–75. https://doi.org/10.1016/j.amjcard.2015.02.038
- Hlatky MA, Boothroyd DB, Bravata DM et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet 2009;373:1190–7. http://dx.doi.org/10.1016/S0140-6736(09)60552-3
- Serruys PW, Morice MC, Kappetein AP et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961–72. http://dx.doi.org/10.1056/NEJMoa0804626
- Kappetein AP, Feldman TE, Mack MJ et al. Comparison of coronary bypass surgery with drug-eluting stenting for the treatment of left main and/or three-vessel disease: 3-year follow-up of the SYNTAX trial. Eur Heart J 2011;32:2125–34. http://dx.doi.org/10.1093/eurheartj/ehr213
- Head SJ, Davierwala PM, Serruys PW, et al. Coronary artery bypass grafting vs. percutaneous coronary intervention for patients with three-vessel disease: final five-year follow-up of the SYNTAX trial. Eur Heart J 2014;35:2821-30. https://dx.doi.org/10.1093/eurheartj/ehu213
- Stone GW, Sabik JF, Surruys PW, et al. Everolimus-eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med 2016;375:2223–35. https://dx.doi.org/10.1056/NEJMoa1610227
- Shlofmitz E, Genereux P, Chen S, et al. Left main coronary artery disease revascularization according to the SYNTAX score. Circulation Cardiovasc Intervent 2019;12: https://doi.org/10.1161/CIRCINTERVENTIONS.118.008007
- Stone GW, Kappetein, Sabik JF et al. for the EXCEL Trial Investigators. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med 2019 (published online 28th September 2019). https://dx.doi.org/10.1056/NEJMoa1909406
- Roques F, Nashef SA, Michel P et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19,030 patients. Eur J Cardiothorac Surg 1999;15:816–22; discussion 822–3. http://dx.doi.org/10.1016/S1010-7940(99)00106-2
- Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg 2008;135:180–7. http://dx.doi.org/10.1016/j.jtcvs.2007.09.011
- Tu JV, Ko DT, Guo H et al. Determinants of variations in coronary revascularization practices. CMAJ 2011. http://doi.org/10.1503/cmaj.111072
- Collet C, Onuma Y, Andreini D, et al. Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur Heart J 2018;39:3689-98. https://dx.doi.org/10.1093/eurheartj/ehy581
- Fox KA. COURAGE to change practice? Revascularisation in patients with stable coronary artery disease. Heart 2009;95:689–92. http://dx.doi.org/10.1136/hrt.2009.167148
- Lin GA, Dudley RA, Lucas FL, Malenka DJ, Vittinghoff E, Redberg RF. Frequency of stress testing to document ischemia prior to elective percutaneous coronary intervention. JAMA 2008;300:1765–73. http://dx.doi.org/10.1001/jama.300.15.1765

All rights reserved. No part of this programme may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publishers, Medinews (Cardiology) Limited.
It shall not, by way of trade or otherwise, be lent, re-sold, hired or otherwise circulated without the publisher’s prior consent.
Medical knowledge is constantly changing. As new information becomes available, changes in treatment, procedures, equipment and the use of drugs becomes necessary. The editors/authors/contributors and the publishers have taken care to ensure that the information given in this text is accurate and up to date. Readers are strongly advised to confirm that the information, especially with regard to drug usage, complies with the latest legislation and standards of practice.
Healthcare professionals should consult up-to-date Prescribing Information and the full Summary of Product Characteristics available from the manufacturers before prescribing any product. Medinews (Cardiology) Limited cannot accept responsibility for any errors in prescribing which may occur.