Introduction
The first detailed account of the medical use of digoxin was in English physician William Withering’s 1785 publication, An account of the foxglove and some of its medical uses: with practical remarks on dropsy and other diseases, in which he described using the leaves of Digitalis purpurea (common foxglove) to treat patients with dropsy (anasarca i.e., gross generalised oedema), hydrothorax (pleural effusions), and breathlessness – now commonly recognised as the heart failure (HF) syndrome. He noted the urine output of some patients would greatly increase and with it, their condition would improve. In others he noted what we now recognised as some of the symptoms of digoxin toxicity: nausea, vomiting, visual changes, and bradycardia.1
Despite over 200 years of research, it remains unclear what role digoxin should have in patients with HF and the use of digoxin in clinical practice is highly variable. There are two common clinical scenarios in which digoxin may be used, namely, ventricular rate control for patients with atrial fibrillation (AF) and for symptom relief in patients with HF and a reduced ejection fraction (HFrEF).
Digoxin and AF
AF is common in older patients.2 Current National Institute of Health and Care Excellence (NICE) and European Society of Cardiology (ESC) guidelines recommend beta-blockers or rate-limiting calcium channel blockers (verapamil or diltiazem) as first-line treatment for ventricular rate control, with digoxin as second line. However, up to 40% of patients with AF may also have left ventricular systolic dysfunction (LVSD),3 in whom verapamil or diltiazem would be contra-indicated. The recent RATE-AF (Rate Control Therapy Evaluation in Permanent Atrial Fibrillation) trial of digoxin versus bisoprolol for ventricular rate control in patients with permanent AF found no difference in resting heart rate between the two groups with a signal towards improved quality of life with digoxin.4 An important advantage for digoxin is that it is the only medication which can slow ventricular rate in patients with AF without reducing systolic blood pressure.
Digoxin and HFrEF
Digoxin was the cornerstone of treatment for patients with the HF syndrome until the discovery of mercurial and loop diuretics in the 1950s and ‘60s. However, its use has declined since the discovery of inhibitors of the renin-angiotensin-aldosterone system (RAAS) and sympathetic nervous system (figure 1).5–11
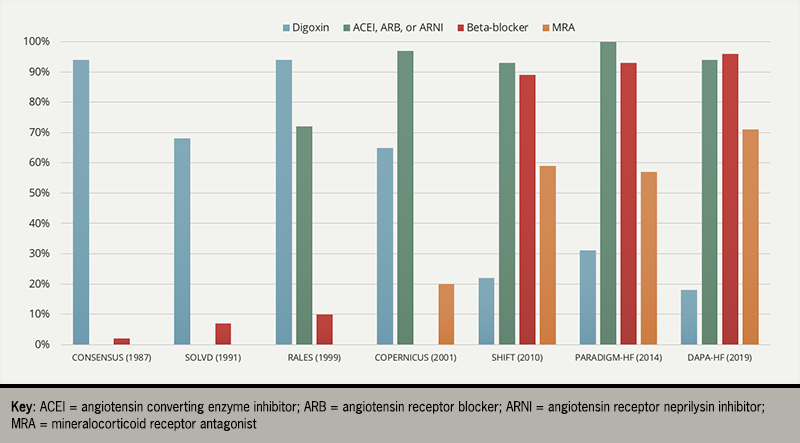
There has only been one large placebo-controlled, randomised controlled trial (RCT) of digoxin in HF – the DIG (Digitalis Investigation Group) trial – which found that digoxin had no effect on the primary endpoint of all-cause mortality compared to placebo in patients with HFrEF who were in sinus rhythm. Digoxin did, however, reduce the risk of hospitalisation with HF (a pre-specified secondary endpoint).12
Currently, approximately 20% of patients with HF take digoxin,13,14 the majority of whom have HF and AF.15 Both the NICE and ESC HF guidelines cautiously recommend digoxin for the treatment of patients with HFrEF in sinus rhythm to reduce the risk of HF hospitalisation.16,17
There have been multiple observational reports which have suggested that digoxin may increase cardiovascular morbidity and mortality.18 Such data may have led to an unfair view that digoxin is unsafe; no data from randomised trials have found any signal of harm with digoxin.15 Additionally, all data on the use of digoxin in patients with HFrEF preceded the widespread use of RAAS or sympathetic nervous system antagonists; we have no data on the benefits (or harms) of digoxin when given alongside modern medical therapy for HF. As with all therapies for which there is little evidence, digoxin has its equally vocal detractors and its proponents. Regardless, it is a commonly used medication with a potentially fatal toxicity profile.
Pharmacology
Digoxin is one of many cardiac glycosides (or cardiotonic steroids – with a typical 4-ring steroidal structure). Digoxin is a positive inotrope, and negative chronotrope; that is, it increases the strength of myocardial contraction and slows the heart. It may also inhibit renin release by inhibiting the action of the sodium-potassium adenosine triphosphatase (Na+/K+ ATPase) pump.
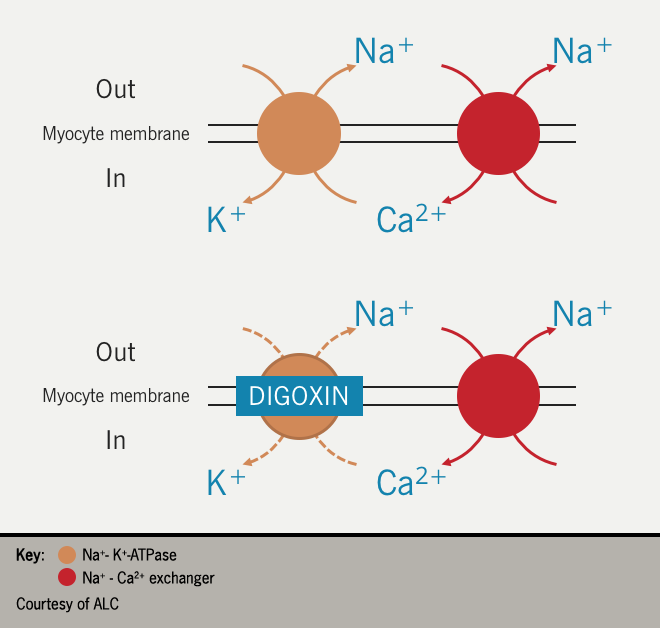
On the plasma membrane of myocytes, the Na+/K+ ATPase removes intracellular Na+ in exchange for K+. Inhibition of the pump increases the intracellular Na+ concentration, which, in turn, increases the activity of the sodium-calcium (Na+/Ca2+) exchanger responsible for removing intracellular Na+ in exchange for Ca2+. The consequence is an increase in the intracellular Ca2+ concentration; increased myocyte Ca2+ concentration increases the force of the myocardial contraction i.e., positive inotropy.19
However, this is only one aspect of the incompletely understood mechanism by which digoxin exerts its clinical effects. It is unclear how digoxin mediates its negative chronotropic effects but it is likely due to a combination of activation of the vagus nerve and inhibition of the sympathetic nervous system.20,21 Inhibition of renal Na+/K+ ATPase may reduce Na+ reabsorption, thus reducing renin secretion via tubuloglomerular feedback.22,23
Absorption of oral digoxin occurs in the small intestine and clinical effect can be seen within 2 hours of ingestion (30 minutes, if given intravenously [IV]), with peak effect seen at 4–6 hours. Although digoxin can be metabolised by the liver, the majority is excreted unchanged by the kidneys. Thus, renal function significantly impacts the half-life of digoxin. In a patient with normal renal function, the half-life of digoxin is around 40 hours and steady state is reached in 8–9 days. However, in patients with impaired renal function, the half-life can be as long as 5 days, and thus a steady state will only be reached after 25 days of treatment. The risk of accumulation and toxicity with digoxin is greatly increased in patients with renal dysfunction.
Toxicity
Table 1. Symptoms & signs of digoxin toxicity
| Metabolic |
|---|
| Hyperkalaemia |
| Gastro-intestinal |
| Nausea |
| Vomiting |
| Anorexia |
| Diarrhoea |
| Neurological |
| Yellow-green discolouration or blurring of vision (xanthopsia) |
| Headache |
| Fatigue |
| Weakness |
| Cardiac |
| Bradycardia due to sinus node exit and/or atrioventricular nodal block |
| Hypotension |
| Ventricular ectopy |
| (Non-sustained) ventricular tachycardia |
| Supraventricular tachycardia |
| Cardiac arrest |
As with many medications, the list of symptoms which could constitute digoxin toxicity is long and non-specific (table 1). In the DIG trial, the proportion of patients who reported side effects consistent with digoxin toxicity was 12% in the digoxin group and 8% in the placebo group.12
Prior to the DIG trial, the therapeutic window for serum digoxin concentrations was assumed to be 0.8–2.0 ng/mL. While post-hoc analyses should be interpreted with caution, patients in the DIG trial with serum concentrations >1.2 ng/mL were more likely than those taking placebo or who had lower digoxin concentrations to be admitted with symptoms of digoxin toxicity or to die.24 The therapeutic window should probably be considered to be a bit lower than previously thought (0.6–1.2 ng/mL), but symptoms of toxicity can occur within that range.
How digoxin mediates the non-cardiac toxic effects is poorly understood. Bradycardia and hypotension are likely to be due to excessive activation of the vagus nerve and inhibition of the sympathetic nervous system.
There are two mechanisms by which digoxin toxicity can induce arrhythmia:
- arrhythmic effects of hyperkalaemia (see below)
- excess intracellular Ca2+ accumulation due to inhibition of the Na+/K+ ATPase pump (figure 3).
At therapeutic concentrations, the increased intracellular Ca2+ is sequestered in the endoplasmic reticulum (ER) during diastole. With digoxin toxicity, the ER becomes saturated with Ca2+, which now leaks into the cytoplasm. The rise in intracytoplasmic Ca2+ decreases electronegativity within the myocyte, decreasing transmembrane potential and increasing the chance of depolarisation (delayed after depolarisation – DAD). The DAD may trigger an action potential causing ectopic beat(s) or arrhythmia in the atria or ventricles (figure 3).
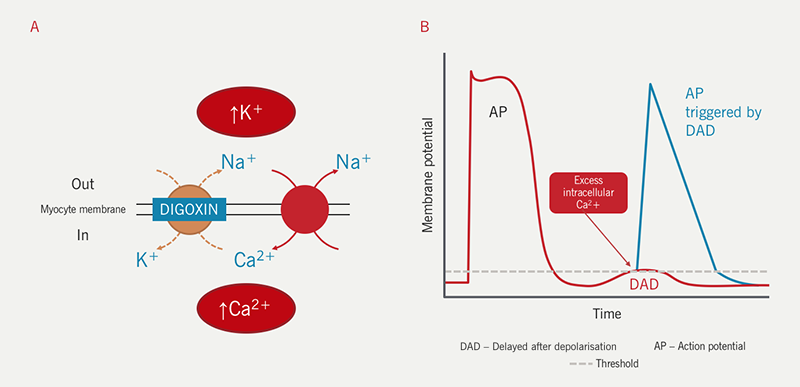
Digoxin toxicity and electrolytes
Digoxin binds to the Na+/K+ ATPase pump at the same site as K+ ions. Thus, in patients with hypokalaemia (and hypomagnesaemia), the affinity of digoxin for the Na+/K+ ATPase pump increases and toxicity is more likely. Hypokalaemia is common in patients with HF, particularly those taking high-dose diuretic therapy.25 While hypokalaemia is not a contraindication for treatment with digoxin, caution is needed.
Similarly, in patients with hypercalcaemia, higher serum concentrations of Ca2+ increase the likelihood of excess intracellular Ca2+ accumulation, increasing the chance of toxicity.
On the other hand, digoxin toxicity can cause electrolyte abnormalities, the most worrisome of which is hyperkalaemia. A high serum K+ concentration in a patient taking digoxin suggests excessive inhibition of the Na+/K+ ATPase pump allowing accumulation of K+ in the serum (figure 3). In turn, this can be arrhythmogenic, thus increasing the risk of fatal arrhythmia in patients with digoxin toxicity.
Digoxin toxicity and concomitant medications
Table 2. Drug interactions in digoxin toxicity
| Medication | Mechanism of increasing the likelihood of digoxin toxicity |
|---|---|
| Diuretics | Hypokalaemia / renal dysfunction |
| RAAS inhibitors | Renal dysfunction |
| Warfarin | Reduced protein binding of digoxin causing increased free serum digoxin concentration |
| Clofibrate | |
| Phenobarbital | |
| Spironolactone | Reduced metabolism or excretion causing increased serum concentration |
| Amiodarone | |
| Propafenone | |
| Diltiazem | |
| Flecainide | |
| Indometacin | |
| Aspirin | |
| Ibuprofen |
There are some drug interactions which can reduce digoxin metabolism and excretion or increase serum concentration of free digoxin (table 2). However, the most common drug interactions associated with digoxin toxicity are those which increase the likelihood of toxicity through other mechanisms (table 2).
Renal dysfunction is a key driver of digoxin toxicity and there are many medications which are known to cause renal dysfunction or prevent recovery of renal function if the deterioration is due to a systemic insult. Diuretics – almost universally used in patients with heart failure – commonly cause hypokalaemia, thus also increasing the likelihood of digoxin toxicity. Digoxin is often prescribed alongside beta-blockers and the combination may also cause excessive bradycardia.
Digoxin toxicity and the ECG
An additional complexity is that digoxin can cause changes to the electrocardiogram (ECG) that are not related to toxicity. These include ventricular ectopic beats; shortening of the QT interval; reduced T wave voltage; and the typical “reverse-tick” or “Salvador Dali moustache sign” (figure 4). The presence of these changes does not necessarily indicate toxicity but should alert the clinician to the possibility of digoxin toxicity should clinical features be present.
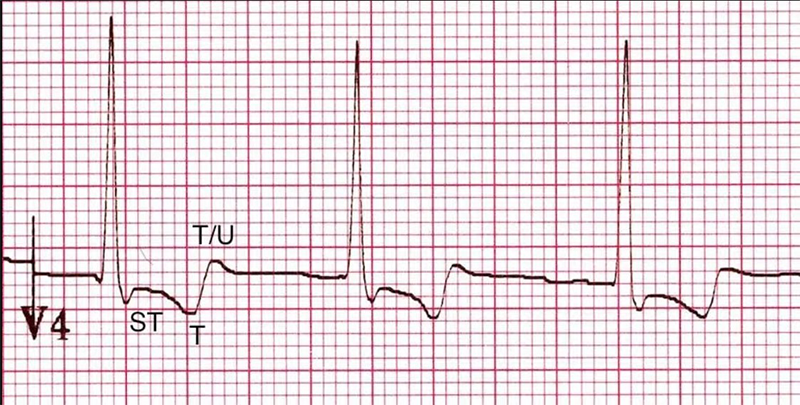
ECG changes associated with toxicity include: T wave inversion; tachy- or bradyarrhythmia; and features of hyperkalaemia, such as peaked T waves, small or absent P waves, increased QRS duration, sine-wave pattern and arrhythmia.
Treating digoxin toxicity
The treatment of digoxin toxicity is mostly supportive and includes identifying and treating the underlying cause, such as renal dysfunction due to infection, medications or hypovolaemia; correcting electrolyte abnormalities and supporting non-life-threatening arrhythmia. However, in some cases, active treatment may be required.
Since digoxin has a high affinity for cardiac and skeletal muscle and renal dysfunction is a common cause of toxicity, dialysis is ineffective. Digoxin immune Fab (marketed as DigiFab™) is the only active treatment available for digoxin toxicity. It is derived from the antigen-binding fragment (Fab) of ovine (sheep) antibodies specific for digoxin. Digoxin immune Fab has a high affinity for digoxin, binding to free digoxin and creating an inactive complex which is freely excreted in the urine (figure 5).
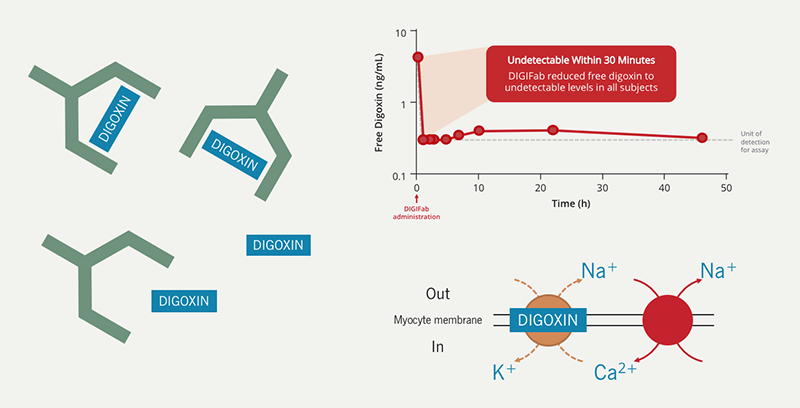
The dose of digoxin immune Fab is determined by either the amount of digoxin ingested or by serum digoxin concentration and body weight, if the dose is not known.
The manufacturer advises that the dose can be given in stages: give half the recommended dose initially and the other half within 2 hours if there is no clinical effect. An 80 kg patient with serum levels of 2.0 ng/mL needs 64 mg of digoxin immune Fab or 2 vials. The medication is reconstituted with water for injection (4 mL per vial) and then diluted in normal saline (0.9% NaCl) and given IV over 30 minutes.26
The presentation of digoxin toxicity can be grouped into three broad categories which guide how digoxin immune Fab is given (table 3).
Table 3. Treating digoxin toxicity
| Acute toxicity | Acute-on-chronic toxicity | Chronic toxicity |
|---|---|---|
| Intentional or accidental overdose in digoxin-naïve patient | Intentional or accidental overdose in a patient taking regular digoxin | Sub-acute accumulation of digoxin to toxic levels in a patient taking regular digoxin who develops a risk factor for digoxin toxicity (e.g., renal dysfunction or hyperkalaemia) |
| Administration of digoxin immune Fab | ||
| Give half the calculated dose over 30 minutes | Give full calculated dose if amount ingested is known | Give half the calculated dose over 30 minutes |
| Give the remaining dose if no response after 2 hours | Give half dose if amount is unknown | Give the remaining dose if evidence of toxicity recurs (rebound) |
In one small study of healthy control patients who had been given 1 mg digoxin IV, serum digoxin levels were undetectable 30 minutes after infusion of digoxin immune Fab given 2 hours after the dose of digoxin (figure 5).27 The serum half-life of digoxin and digoxin immune Fab is similar. Both (and their complex) are excreted by the kidneys.28 While the duration of binding of digoxin immune Fab to free digoxin is unknown, case reports of patients with renal failure have found a “rebound” in serum digoxin concentrations 1–8 days after the administration of digoxin immune Fab,28–30 suggesting that the digoxin immune Fab and digoxin have become unbound before they have been excreted.
Although poorly understood, there are other non-renal routes by which the digoxin immune Fab-digoxin complex can be excreted, which take much longer than renal excretion, for example, intracellular sequestration and degradation,31 or endogenous catabolism, as with other immunoglobulins.32 While the dose of digoxin immune Fab need not be reduced in patients with renal impairment, the period of monitoring after administration should be prolonged to pre-empt and treat any rebound toxicity.
There is currently no evidence base upon which to guide treatment with digoxin immune Fab. An obvious indication is life-threatening arrhythmia but some expert opinion recommends the use of digoxin immune Fab in patients with suspected digoxin toxicity and hyperkalaemia (serum potassium concentrations ≥5.0 mmol/L) on the basis that these patients are at high risk of life-threatening arrhythmia, in which case, prevention is preferable to cure.33,34
Digoxin immune Fab is derived from sheep antibodies and the product also contains extracts from the papaya fruit; hence, there is a risk of allergic reaction and anaphylaxis, although the frequency is unknown. Another common medicinal product derived from sheep is lanolin in emollients. If a patient is known to have lanolin sensitivity, the potential for an allergic or anaphylactic reaction to digoxin immune Fab is presumably greater. However, concerns about a potential allergic reaction are not a contraindication to offering a potentially life-saving treatment in an emergency.29,30
An approach to treating digoxin toxicity
- Symptoms and signs of digoxin toxicity are common and non-specific; a high index of suspicion is required to make the diagnosis.
- A key feature of the history is the timing of ingestion; some patients may present with symptoms of toxicity, others may present reporting an intentional or accidental overdose.
- In patients with suicidal ideation, the overdose may be of more than one drug. The toxic effects of which will also need to be assessed and treated.
- Serum digoxin levels should be taken as early as possible in cases of suspected toxicity.
- The decision to treat may need to be urgent pending the result.
- Infection, renal impairment, electrolyte abnormalities – hyperkalaemia, in particular – should be excluded or treated if present.
- Any patient with known or suspected digoxin toxicity is at a high risk of arrhythmia and thus immediate cardiac monitoring is necessary. Some patients may be taking other medications that might exacerbate the situation, such as beta-blockers, diuretics, or inhibitors of the RAAS – these should be stopped (at least temporarily).
- Patients with suspected digoxin toxicity and hyperkalaemia should receive urgent treatment to correct serum potassium levels. Use of digoxin immune Fab should be considered.
- IV calcium salts are often given to reduce the risk of ventricular arrhythmia in patients with hyperkalaemia. However, in patients with hyperkalaemia due to digoxin toxicity, administration of IV calcium salts paradoxically increases the risk of ventricular arrhythmia. This is because excess intracellular calcium is one mechanism by which digoxin toxicity can cause arrhythmia.
- Patients with suspected or confirmed digoxin toxicity and a haemodynamically stable arrhythmia should be managed as per the Resuscitation Council’s Advanced Life Support protocol.
- Digoxin immune Fab should be available, should the need arise.
- Patients with suspected or confirmed digoxin toxicity with a haemodynamically unstable arrhythmia should be treated as per the Advanced Life Support protocol and be given digoxin immune Fab.
- Patients with digoxin toxicity and end-stage renal failure who have been treated with digoxin immune Fab should have prolonged monitoring and repeat serum digoxin concentrations measured 24–48 hours after administration to detect any rebound in serum digoxin levels.
Summary
Digoxin is commonly used in the treatment of HF and/or AF. It slows the ventricular rate in atrial fibrillation and reduces the need for hospitalisation for patients with heart failure who are in sinus rhythm due to reduced left ventricular ejection fraction. It has a narrow therapeutic window. Most patients taking digoxin are elderly with multiple comorbidities, including renal dysfunction. Many are also likely to take one or more medications that increase the risk of worsening renal function. Early recognition of toxicity is the key to management. Supportive care whilst digoxin is excreted is usually all that is necessary but in the few instances when active treatment of digoxin toxicity is required, digoxin immune Fab is an effective, quick-acting and potentially life-saving treatment.
References
1. Withering W. (1785) An account of foxglove and some of its medical uses: with practical remarks on dropsy and other disease. London: GGJ & J Robinson.
2. DeWilde S, Carey IM, Emmas C, Richards N, Cook DG. Trends in the prevalence of diagnosed atrial fibrillation, its treatment with anticoagulation and predictors of such treatment in UK primary care. Heart 2006;92(8):1064–70. https://doi.org/10.1136/hrt.2005.069492
3. Cha YM, Redfield MM, Shen WK, Gersh BJ. Atrial fibrillation and ventricular dysfunction: a vicious electromechanical cycle. Circulation 2004;109(23):2839–43. https://doi.org/10.1161/01.CIR.0000132470.78896.A8
4. Kotecha D, Bunting KV, Gill SK, et al. Effect of digoxin vs bisoprolol for heart rate control in atrial fibrillation on patient-reported quality of life: the RATE-AF randomized clinical trial. JAMA 2020;324(24):2497–508. https://doi.org/10.1001/jama.2020.23138
5. CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987;316(23):1429–35. https://doi.org/10.1056/NEJM198706043162301
6. SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB Jr, Cohn JN. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions [published correction appears in N Engl J Med 1992;327(24):1768]. N Engl J Med 1992;327(10):685–91. https://doi.org/10.1056/NEJM199209033271003
7. Pitt B, Zannad F, Remme WJ, et al. Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341(10):709–17. https://doi.org/10.1056/NEJM199909023411001
8. Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344(22):1651–8. https://doi.org/10.1056/NEJM200105313442201
9. Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study [published correction appears in Lancet 2010;376(9757):1988]. Lancet 2010;376(9744):875–85. https://doi.org/10.1016/S0140-6736(10)61198-1
10. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371(11):993–1004. https://doi.org/10.1056/NEJMoa1409077
11. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381(21):1995–2008. https://doi.org/10.1056/NEJMoa1911303
12. Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336(8):525–33. https://doi.org/10.1056/NEJM199702203360801
13. National Institute for Cardiovascular Outcomes Research. National Heart Failure Audit 2020/21 Summary Report. 2021. Available from: NICOR | Heart Failure (Heart Failure audit) https://www.nicor.org.uk/heart-failure-heart-failure-audit/ [accessed 09/04/2023]
14. Crespo-Leiro MG, Anker SD, Maggioni AP, et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions [published correction appears in Eur J Heart Fail 2017;19(3):438]. Eur J Heart Fail 2016;18(6):613–25. https://doi.org/10.1002/ejhf.566
15. Veenis JF, Brunner-La Rocca HP, Linssen GCM, et al. Atrial fibrillation in chronic heart failure patients with reduced ejection fraction: The CHECK-HF registry. Int J Cardiol 2020;308:60–6. https://doi.org/10.1016/j.ijcard.2020.03.001
16. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure [published correction appears in Eur Heart J 2021;422(48):4901]. Eur Heart J 2021;42(36):3599–726. https://doi.org/10.1093/eurheartj/ehab368
17. National Institute of Health and Care Excellence. Chronic Heart Failure in Adults: Diagnosis and Management. NICE Guideline NG106. 2018. Available from: Overview | Chronic heart failure in adults: diagnosis and management | Guidance | NICE https://www.nice.org.uk/guidance/ng106 [accessed 09/04/2023]
18. Ziff OJ, Lane DA, Samra M, et al. Safety and efficacy of digoxin: systematic review and meta-analysis of observational and controlled trial data [published correction appears in BMJ 2015;351:h4937]. BMJ 2015;351:h4451. https://doi.org/10.1136/bmj.h4451
19. Clark AL. Gardener RS. McDonagh TA. (2022) Oxford Textbook of Heart Failure. Second edition. Oxford: Oxford University Press.
20. Ten Eick RE, Hoffman BF. Chronotropic effect of cardiac glycosides in cats, dogs, and rabbits. Circ Res 1969;25(4):365–78. https://doi.org/10.1161/01.res.25.4.365
21. Chai CY, Wang HH, Hoffman BF, Wang SC. Mechanisms of bradycardia induced by digitalis substances. Am J Physiol 1967;212(1):26–34. https://doi.org/10.1152/ajplegacy.1967.212.1.26
22. Torretti J, Hendler E, Weinstein E, Longnecker RE, Epstein FH. Functional significance of Na- K-ATPase in the kidney: effects of ouabain inhibition. Am J Physiol 1972;222(6):1398–405. https://doi.org/10.1152/ajplegacy.1972.222.6.1398
23. Covit AB, Schaer GL, Sealey JE, Laragh JH, Cody RJ. Suppression of the renin-angiotensin system by intravenous digoxin in chronic congestive heart failure. Am J Med 1983;75(3):445–7. https://doi.org/10.1016/0002-9343(83)90346-7
24. Rathore SS, Curtis JP, Wang Y, Bristow MR, Krumholz HM. Association of serum digoxin concentration and outcomes in patients with heart failure. JAMA 2003;289(7):871–8. https://doi.org/10.1001/jama.289.7.871
25. Savarese G, Xu H, Trevisan M, et al. Incidence, predictors, and outcome associations of dyskalemia in heart failure with preserved, mid-range, and reduced ejection fraction. JACC Heart Fail 2019;7(1):65–76. https://doi.org/10.1016/j.jchf.2018.10.003
26. National Institute for Health and Care Excellence. British National Formulary. Digoxin-specific antibody. Available from: Digoxin-specific antibody | Drugs | BNF | NICE. https://bnf.nice.org.uk/drugs/digoxin-specific-antibody/ [Accessed 09/04/2023]
27. Ward SB, Sjostrom L, Ujhelyi MR. Comparison of the pharmacokinetics and in vivo bioaffinity of DigiTAb versus Digibind. Ther Drug Monit 2000;22(5):599–607. https://doi.org/10.1097/00007691-200010000-00016
28. Ujhelyi MR, Robert S, Cummings DM, et al. Disposition of digoxin immune Fab in patients with kidney failure. Clin Pharmacol Ther 1993;54(4):388–94. https://doi.org/10.1038/clpt.1993.165
29. Allen NM, Dunham GD, Sailstad JM, Findlay JW. Clinical and pharmacokinetic profiles of digoxin immune Fab in four patients with renal impairment. DICP 1991;25(12):1315–20. https://doi.org/10.1177/106002809102501205
30. Ujhelyi MR, Robert S, Cummings DM, et al. Influence of digoxin immune Fab therapy and renal dysfunction on the disposition of total and free digoxin. Ann Intern Med 1993;119(4):273–77. https://doi.org/10.7326/0003-4819-119-4-199308150-00004
31. Schaumann W, Kaufmann B, Neubert P, Smolarz A. Kinetics of the Fab fragments of digoxin antibodies and of bound digoxin in patients with severe digoxin intoxication. Eur J Clin Pharmacol 1986;30(5):527–33. https://doi.org/10.1007/BF00542410
32. Wochner RD, Strober W, Waldmann TA. The role of the kidney in the catabolism of Bence Jones proteins and immunoglobulin fragments. J Exp Med 1967;126(2):207–21. https://doi.org/10.1084/jem.126.2.207
33. Kanji S, MacLean RD. Cardiac glycoside toxicity: more than 200 years and counting. Crit Care Clin 2012;28(4):527–35. https://doi.org/10.1016/j.ccc.2012.07.005
34. Pincus M. Management of digoxin toxicity. Aust Prescr 2016;39(1):18–20. https://doi.org/10.18773/austprescr.2016.006

All rights reserved. No part of this programme may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publishers, Medinews (Cardiology) Limited.
It shall not, by way of trade or otherwise, be lent, re-sold, hired or otherwise circulated without the publisher’s prior consent.
Medical knowledge is constantly changing. As new information becomes available, changes in treatment, procedures, equipment and the use of drugs becomes necessary. The editors/authors/contributors and the publishers have taken care to ensure that the information given in this text is accurate and up to date. Readers are strongly advised to confirm that the information, especially with regard to drug usage, complies with the latest legislation and standards of practice.
Healthcare professionals should consult up-to-date Prescribing Information and the full Summary of Product Characteristics available from the manufacturers before prescribing any product. Medinews (Cardiology) Limited cannot accept responsibility for any errors in prescribing which may occur.