Highlights of the European Society of Cardiology (ESC) 2011 Congress, held in Paris, France, from 27th–31st August, included encouraging results with the third new oral anticoagulant alternative to warfarin for patients with atrial fibrillation, a treatment for recurrent endocarditis, and the suggestion that six months dual antiplatelet therapy may be enough after drug-eluting stent placement.
ARISTOTLE: apixaban superior to warfarin in AF patients
Another oral anticoagulant has shown good results in comparison to warfarin for use in the prevention of stroke in patients with atrial fibrillation (AF). The new oral factor Xa inhibitor, apixaban, was superior to warfarin in preventing stroke or systemic embolism and was also associated with less bleeding and lower mortality than warfarin in the ARISTOTLE trial.
Apixaban is the third of the new generation of oral anticoagulants to be tested in this indication, and seems to have performed the best. The other two agents – dabigatran and rivaroxaban – have also been shown to be viable alternative agents to warfarin in the RE-LY and ROCKET-AF trials, respectively, but apixaban is the only one to have shown significant reductions in stroke, bleeding, and mortality. All three new agents have, however, shown a large reduction in intracranial haemorrhage versus warfarin, prompting commentators at the ESC press conference on ARISTOTLE to agree that any of the new drugs seemed preferable to keeping patients on warfarin.
Presenting the ARISTOTLE results, Dr Christopher Granger (Duke University, US) explained that finding the right dose of these agents was the key to success, something that this trial seemed to have got right. “We seem to have hit the sweet spot on the dose of apixaban, which produced both great efficacy and safety,” he said.
The ARISTOTLE trial randomised 18,201 patients with AF to apixaban (5 mg orally twice daily) or warfarin (target INR of 2.0 to 3.0). After a median follow-up of 1.8 years, results (see table 1) showed that apixaban was associated with a 21% reduction in the risk of stroke or systemic embolism, a 31% reduction in bleeding, and an 11% reduction in all-cause mortality.
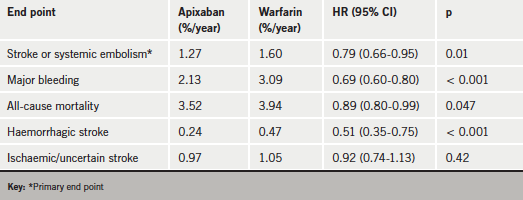
In comparison, the higher dose of dabigatran (150 mg twice daily) tested in RE-LY produced a reduction in stroke but a similar rate of bleeding and an increase in gastrointestinal bleeding compared with warfarin. And the lower dabigatran dose (110 mg twice daily) showed a similar stroke rate to warfarin with less bleeding. The ARISTOTLE investigators are therefore positioning apixaban as “somewhere in between the two dabigatran doses”.
The ROCKET-AF trial with rivaroxaban was more difficult to interpret, with rivaroxaban not being shown superior to warfarin in preventing stroke in the primary intention-to-treat analysis, but achieving significant superiority in an “as-treated” analysis. Major bleeding was similar with rivaroxaban and warfarin, but there was less intracranial and fatal bleeding with rivaroxaban, which also has the practical advantage over the other two drugs of a once-daily dose.
The ARISTOTLE results have since been published in The New England Journal of Medicine (N Engl J Med 2011;365:981–92). In the paper, the investigators report that for every 1,000 patients treated for 1.8 years, apixaban, as compared with warfarin, prevented four haemorrhagic strokes, two ischaemic strokes, major bleeding in 15 patients, and death in eight patients.
PRODIGY: six months clopidogrel enough after stenting
Two years of dual antiplatelet therapy after coronary stenting was no better than six months in preventing cardiac events but was associated with significantly more bleeding in the PRODIGY (Prolonging Dual Antiplatelet Treatment After Grading Stent-induced Intimal Hyperplasia) trial.
Presenting the data, Dr Marco Valgimigli (University Hospital of Ferrara, Italy) said the results questioned the current guidelines which recommended at least 12 months’ dual antiplatelet therapy after drug-eluting stenting. “Our study clearly shows that the benefit to risk ratio of prolonged therapy has been over-emphasised,” he stated.
In the study, 2,000 patients scheduled for elective or urgent stenting were randomised to receive one of three different drug-eluting stents or a third-generation bare-metal stent. At 30 days, patients in each stent group were then further randomised to either six or 24 months of dual antiplatelet treatment (clopidogrel plus aspirin).
Results showed that the primary outcome (all-cause mortality, non-fatal myocardial infarction or cerebrovascular accident) at two years was almost identical in patients given six months and in those given 24 months of treatment. However, the longer treatment group had double the risk of bleeding events (defined as the Bleeding Academic Research Consortium classification). The risks of TIMI-defined major bleeding and transfusions were also increased in the 24-month clopidogrel group. There was no difference in results according to which stent was used.
Dr Valgimigli said the study had important economic implications, as the longer term treatment was also associated with more hospitalisations (to treat bleeding) and more diagnostic and therapeutic resources.
Colchicine effective for preventing pericarditis
Colchicine appears to be a safe low-cost drug for rapid symptom relief, improved remission rates and reduced recurrence after an initial episode of recurrent pericarditis, according to the results of the CORP (Colchicine for Recurrent Pericarditis) trial.
Presenting the data, Dr Massimo Imazio (Maria Vittoria Hospital, Turin, Italy) explained that recurrences of pericarditis were relatively common, occurring in about 20–50% of patients who have a first episode.
While there has been some data from observational studies and small open-label randomised studies suggesting that colchicine may be a safe and useful drug for preventing recurrences, this is the first multi-centre, double-blind, randomised, placebo-controlled trial to look at this issue.
The CORP trial included 120 patients with a first episode of recurrent pericarditis from four centres in Italy. Colchicine was added to standard anti-inflammatory therapy, and was given at an initial dose of 1.0–2.0 mg for the first day and a maintenance dose of 0.5–1.0 mg daily for the following six months. A lower dose was used in patients under 70 kg in weight or intolerant of the higher dose.
Results (table 1) showed that colchicine significantly reduced the incidence of recurrences at 18 months, the primary end point compared with placebo. The colchicine group also had a lower incidence of symptom persistence at 72 hours, fewer recurring episodes, a higher rate of remission at one week, and longer time to a subsequent recurrence. The rate of side effects was similar in both groups.
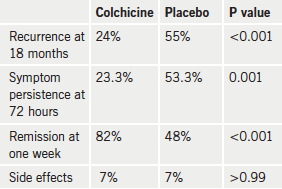
Full results have been published in the Annals of Internal Medicine (Ann Intern Med 2011;155:409–14).
Latest from ASCOT: could statins reduce death from infections?
Long-term results of the primary prevention ASCOT-LLA study suggest that statins may reduce death from infection.
ASCOT-LLA (Anglo-Scandinavian Cardiac Outcomes Trial – Lipid-Lowering Arm) studied atorvastatin 10 mg in patients with hypertension, but with no established coronary heart disease (CHD) or high cholesterol levels. The main results reported in 2003, showed that atorvastatin reduced fatal CHD and non-fatal myocardial infarction after a median follow-up of 3.3 years. At the time the study was stopped, there was a non-significant trend toward reduction in all-cause mortality.
After the study ended, mortality data continued to be collected in patients originally randomised to atorvastatin or placebo for a median of 11 years. Results from this extended follow-up show that all-cause mortality was significantly reduced by 14%, and non-cardiovascular mortality was significantly reduced by 15%, but there was no difference in cardiovascular deaths. In addition, the reduction in non-cardiovascular deaths was driven by a significant 36% reduction in deaths due to infection and respiratory illness.
Presenting the data, Dr Peter Sever (Imperial College, London) said that preclinical studies suggested that statins may indeed have some anti-infective actions, and other observational studies have shown reduced mortality from sepsis with statins.
He added that these results should be viewed as hypothesis generating, and called for a prospective study of statins in patients at high risk for infection to see if they could reduce sepsis or death from infection.
These latest results from ASCOT have been published online in the European Heart Journal (Eur Heart J 2011; doi:10.1093/eurheartj/ehr333)
CRISP: no benefit of counterpulsation in MI patients not in shock
Routine counterpulsation with an intra-aortic balloon pump during percutaneous coronary intervention (PCI) for large anterior myocardial infarction (MI) does not reduce infarct size, according to results of the CRISP-AMI study.
The investigators, led by Dr Manesh Patel (Duke University, US) suggest that such counterpulsation should be reserved for MI patients who develop signs of haemodynamic instability.
In the trial, 337 patients undergoing primary PCI for an anterior ST-elevation MI but who were not in shock were randomised to counterpulsation support or no such support. Infarct size four days later was not significantly different between the two groups.
The findings have also now been published in JAMA (JAMA 2011;306: 1329–37).
Remote ICD monitoring
looks good
Two new studies have suggested that ICD (implantable cardioverter-defibrillator) patients monitored remotely have fewer inappropriate shocks than patients returning to hospital for checks, without any significant difference in clinical events.
In the EVATEL study, presented by Dr Philippe Mabo (University Hospital, Rennes, France), 1,501 ICD patients were randomised to conventional checks at the implanting centre or remote transmission of data from the ICD to the implant centre. Major clinical events were similar in both groups, and fewer patients in the remotely monitored group experienced inappropriate or ineffective therapy from their ICD (4.7% vs. 7.5%, p=0.03).
The second study, known as ECOST, was presented by Dr Salem Kacet (Regional University Hospital, Lille, France). In this study, 433 patients were randomised to either daily remote monitoring or traditional clinic visits.
Results showed that the remote-monitoring patients were about half as likely as the clinic patients to receive an inappropriate ICD shock (5% vs. 10.4%, p=0.03), which resulted in a significantly longer ICD battery life in the remotely monitored patients. There were also fewer hospitalisations in the remote group (3 vs. 11, p=0.02).
dal-VESSEL: new CETP inhibitors lack side-effects of torcetrapib
A new high-density lipoprotein cholesterol (HDL-C) raising drug in development appears not to be associated with the problems that may have caused the downfall of the first such agent in this class, torcetrapib, according to a phase 2 study.
The new cholesteryl ester transfer protein (CETP) inhibitor – dalcetrapib – did not impair endothelial function or increase blood pressure, and was generally well tolerated in patients with or at risk of coronary heart disease in the dal–VESSEL study.
Presenting the data, Dr Thomas Lüscher (University Hospital, Zurich, Switzerland) said: “Results so far suggest that dalcetrapib does not have pro-inflammatory or pro-atherogenic effects, does not affect blood pressure and is generally well tolerated by patients treated for up to two years.”
Dal-VESSEL involved 476 patients with HDL-C levels <50 mg/dL who were randomised to dalcetrapib 600 mg/day or placebo in addition to their existing treatments. Results showed that dalcetrapib reduced CETP activity by almost 50% and increased HDL by 31% without changing nitric-oxide-dependent endothelial function or markers of inflammation and oxidative stress.
Dr Lüscher said dalcetrapib raises HDL by a different mechanism to other CETP inhibitors, and has been associated with the efflux of cholesterol from cells in laboratory studies. It is currently being tested in phase 3 trials.
Huge under-use of cheap and proven drugs in heart disease
A new study has shown massive under-use of proven therapies for the secondary prevention of cardiovascular disease.
The PURE (Prospective Urban Rural Epidemiological) study, presented by Dr Salim Yusuf (McMaster University, Canada), collected data on 154,000 adults with a history of heart disease or stroke from 17 countries.
“We found extremely low rates of use of proven therapies in all countries, but these were more marked in middle and low income countries. The study indicates a large gap in secondary prevention globally,” Dr Yusuf said. “There is an urgent need for systematic approaches to understand and solve the causes of the large treatment gap in secondary prevention in all communities. This is a global tragedy and represents a huge wasted opportunity to help millions of people with heart disease at very low cost,” he added.
Results showed large variations in use of proven therapies between high income and low income countries, with statins being used 20 times more often in richer countries. The cheap and widely available aspirin was seven times more likely to be taken in better off nations. But even in the three high income countries studied (Canada, Sweden and the United Arab Emirates), significant numbers of post-MI and stroke patients not taking preventive treatment were found.
Other countries involved in the study were: Argentina, Brazil, Chile, Malaysia, Poland, South Africa, Turkey (upper middle income), China, Colombia, Iran (lower middle income), Bangladesh, India, Pakistan, Zimbabwe (low income).
Dr Yusuf said the results (table 1) showed that most systems in the world did not have an organised approach to secondary prevention, adding that doctors should delegate the role of prevention.

