This case report invites discussion on the challenges of the management of extensive thromboembolism despite standard anticoagulation.
A previously healthy 49-year-old male had an acute pulmonary embolism (PE) and was managed with rivaroxaban anticoagulation and an inferior vena cava (IVC) filter implantation. This patient re-presented with occlusion of his IVC filter with extensive thrombus extending down to his femoral veins bilaterally.
We performed catheter-directed thrombolysis using the EKOSonic Endovascular system. The patient had invasive monitoring alongside use of peri-operative cardiac imaging (TOE). A valvuloplasty balloon was used to prevent upward migration of thrombus.
There is potential for the wider use of the EKOSonic Endovascular system and catheter-directed thrombolysis in centres where there is surgical support available, and in selected patients where there is extensive thrombotic burden with risk of recurrence or long-term complications.
Introduction
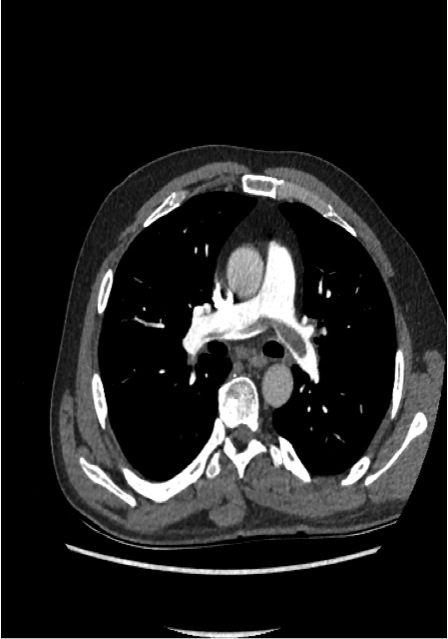
A previously fit and well 49-year-old male presented to our department with acute shortness of breath and associated pleuritic pain. He had pulmonary embolisms (PEs) confirmed on a computed tomography pulmonary angiogram (CTPA) but did not meet the criteria for thrombolysis (figure 1). He was initiated on anticoagulation therapy. In addition, he had evidence of marked eosinophilia, which was felt to be a reflection of his pro-thrombotic state rather than a primary hypereosinophilic condition. An inferior vena cava (IVC) filter was also implanted, after multi-disciplinary team (MDT) discussion; it was felt to be beneficial due to the marked eosinophilia and predicted need for invasive tests and, hence, interruption to anticoagulation. There were plans for full investigation into the underlying cause of the PEs at an appropriate stage.
Not long after discharge from hospital, he re-presented with bilateral lower limb swelling and back pain. A repeat computed tomography (CT) scan confirmed an occluded IVC filter with extensive thrombus distal to this bilaterally. This patient was at risk of further deterioration and he was transferred to a tertiary centre for advanced management. Such extensive thrombus burden meant that usual systemic thrombolysis was not the optimal treatment option given that there was a risk of recurrence, and also of long-term complications, including post-thrombotic syndrome.1 This patient had ultrasound-assisted, catheter-directed thrombolysis (EKOSonicEndoWave Infusion Catheter System2) after a cross-site multi-disciplinary discussion.
Catheter-directed thrombolysis (CDT) is established as an effective treatment modality in selected patients.3 As an example, it is suitable for younger patients with acute proximal deep vein thromboses (DVTs) at risk of PE and post-thrombotic syndrome (PTS). There is an optimal timing in which this treatment should take place, as fresh thrombotic occlusions will respond better with this intervention (i.e. venous recanalisation and reduce incidence of PTS) and based on the available research data, most institutions follow a timing of 12–14 days from diagnosis to CDT treatment. EKOS is an ultrasound system, which improves the efficacy of CDT.4 By using ultrasound energy application, there is mechanical thrombus breakdown, which increases the available surface area for the thrombolytic agent’s action. There is reduction in the infusion time and the total thrombolytic dose administered while, at the same time, achieving good results with venous recanalisation.4
Methods
The procedure was performed under general anaesthesia. First, a transoesophageal echocardiograph (TOE) was performed, which confirmed an intact inter-atrial septum. The right atrium (RA) and right ventricle (RV) were of normal size and function, with no significant valvular pathology. The probe was left in situ to monitor any changes to the right heart that might indicate development of a haemodynamically significant PE from upward migration of emboli.
A 9Fr sheath was placed in his left internal jugular vein (IJV) and a J wire was placed down the RA into the IVC just above the origin of the IVC filter. Through this wire a 30 × 40 mm valvuloplasty balloon was placed and secured in the IVC. The purpose of this was to prevent upward migration of emboli from the distal venous circulation.
The patient was temporarily in a prone position for access of the popliteal veins using ultrasound guidance. Eventually, 6Fr sheaths were placed using a MPA catheter and a Terumo wire. A venogram was performed, confirming occlusive thrombus in the iliac and femoral veins. The sheaths were then exchanged for EKOS catheters using an Amplatz Super Stiff wire.
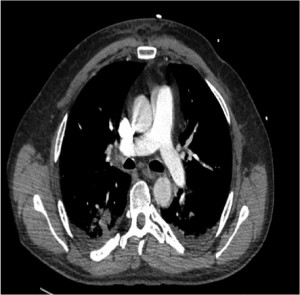
At the end of the procedure, the valvuloplasty balloon was removed and the post-procedural TOE was satisfactory. Intravenous heparin was infused through the EKOS catheters at 10,000 IU over 24 hours. Alteplase was given through the catheters with a total of 20 mg over 20 hours. Unfortunately, on day 1 post-procedure, one of the catheters was malfunctioning and had to be changed. After this process, there was haemodynamic compromise, which we suspect was due to emboli formation with temporary pulmonary outflow obstruction. The parameters spontaneously resolved, however, this led to slight prolongation in total thrombolytic infusion time and dose.
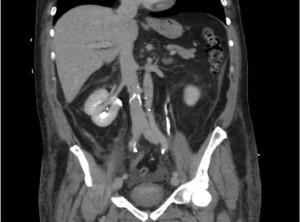
A Swan Ganz catheter was used for monitoring of his pulmonary artery (PA) pressures. On day 3 he had a venogram, which showed complete recanalisation of the femoral and iliac veins and an unobstructed IVC. Post-operative CTs pre-discharge showed absence of emboli (see figures 2 and 3).
The heparin infusion was switched to subcutaneous enoxaparin 1 mg/kg twice daily and then warfarin. Clopidogrel 75 mg once daily was used alongside the anticoagulation throughout. This anticoagulant regimen was devised locally with support from our haematology team.
Summary
This case illustrates recurrent and extensive thromboembolism, despite standard anticoagulation, and we reported how we managed this successfully. There were never any issues with drug compliance and rivaroxaban was taken with food (rivaroxaban bioavailability is significantly reduced if not taken with meals5). The patient had steroid therapy for the secondary hypereosinophilia, alongside anticoagulation. After the events as described, his long-term anticoagulation regimen was changed to warfarin and clopidogrel, but we are aware that there is no national consensus on what the optimal treatment regimen is in such a situation. The recurrent venous thromboembolism would have occurred even without the IVC filter given the extent of thrombotic burden.
EKOSonic catheter-directed thrombolysis is not practised widely across the UK, despite evidence supporting its use if a patient is deemed to be a potential candidate for CDT.4 The use of cardiac imaging is vital for monitoring purposes. The use of a valvuloplasty balloon should have been extended when changing the catheter in this case.
In cases similar to this, the role of the cardiologist will be to ensure safe measures to avoid or manage cardiorespiratory compromise alongside multi-disciplinary discussions and decision making.
Follow-up
This patient recovered fully and has completed an exercise-tolerance test three months after the procedure. His IVC filter has also been removed now. There had been no bleeding complications. No underlying cause for the venous thromboembolism has been found so far despite extensive investigations.
Conclusion
This is a case of thrombus formation despite anticoagulation therapy. There is no formal guideline about how to approach this clinical scenario in current literature. CDT is still not practised widely but there is potential for wider use of the EKOSonic Endovascular system and catheter-directed thrombolysis in selected patients where there is surgical support available.
Acknowledgements
Thanks to the following people/teams for their clinical roles in the case: Intensive Care Unit, The Heart Hospital; Dr Liz Ashley, Cardiac Anaesthetist, The Heart Hospital; Dr Julian Hague Interventional Radiologist, The Heart Hospital; Dr Simon Weston-Smith; Consultant Haematologist, Conquest Hospital, Hastings; Dr Robert Gerber, Consultant Cardiologist, Conquest Hospital, Hastings; Dr John Giles, Consultant Interventional Radiologist, Conquest Hospital, Hastings.
Conflict of interest
None declared.
Key messages
- Recurrent thromboembolism can occur despite standard anticoagulation. The indications for advanced therapy are based on individual case assessment alongside current evidence and guidance
- The cardiologist has an important role in the advanced management of venous thromboembolism including the utilisation of cardiac imaging and right heart catheterisation
- The EKOSonic system can reduce the dose of thrombolytic agents used and so lower risk of haemorrhagic complications
- Additional use of a valvuloplasty balloon can prevent upward embolic complications when appropriate
References
1. Baldwin MJ, Moore HM, Rudarakanchana N, Gohel M, Davies AH. Post-thrombotic syndrome: a clinical review. J Thromb Haemost 2013;11:795–805. http://dx.doi.org/10.1111/jth.12180
2. Dumantepe M, Tarhan A, Yurdakul I, Özler A. US-accelerated catheter-directed thrombolysis for the treatment of deep venous thrombosis. Diagn Interv Radiol 2013;19:251–8. http://dx.doi.org/10.5152/dir.2012.004
3. Patterson BO, Hinchliffe R, Loftus IM, Thompson MM, Holt PJE. Indications for catheter-directed thrombolysis in the management of acute proximal deep venous thrombosis. Arterioscler Thromb Vasc Biol 2010;30:669–74. http://dx.doi.org/10.1161/ATVBAHA.109.200766
4. Owens CA. Ultrasound-enhanced thrombolysis: EKOS EndoWave Infusion Catheter System. Semin Intervent Radiol 2008;25:37–41. http://dx.doi.org/10.1055/s-2008-1052304
5. Stampfuss J, Kubitza D, Becka M, Mueck W. The effect of food on the absorption and pharmacokinetics of rivaroxaban. Int J Clin Pharmacol Ther 2013;51:549–61. http://dx.doi.org/10.5414/CP201812
