A record-breaking meeting of the European Society of Cardiology (ESC) was held in London this year with over 37,000 delegates from all over the world attending. There were 28 clinical presentations at Hot Line sessions, 18 clinical trial updates, 20 registry studies, five new ESC guidelines published, and 4,533 abstracts presented during the five-day meeting from 29th August–2nd September 2015. We present its highlights.
Hypertension highlights
PATHWAY: spironolactone ‘unequivocal’ in treatment of resistant hypertension
The use of spironolactone is overwhelmingly the most effective treatment for resistant hypertension, results from the PATHWAY study have shown.
The study found spironolactone, which has been available for over 50 years, controlled blood pressure (BP) in almost 60% of patients with resistant hypertension and was three times as likely to be the patient’s best drug compared to doxazosin or bisoprolol.
The results were “unequivocal” in favour of spironolactone said the British Hypertension Society investigators. Speaking at the Hot Line session to announce the results, Professor Bryan Williams (University College London, and the British Hypertension Society Research Network) said: “Spironolactone is the most effective treatment for resistant hypertension, and these results should influence treatment guidelines globally.”
Resistant hypertension − defined as uncontrolled BP despite treatment with at least three BP-lowering drugs − is estimated to occur in about 10% of hypertensive patients. Professor Williams said patients should not now be defined as having resistant hypertension unless their BP remained uncontrolled on spironolactone.
The PATHWAY (Prevention and Treatment of Hypertension with Algorithm Based Therapy) study, funded by the British Heart Foundation, looked at whether additional diuretic therapy with spironolactone would be the most effective at reducing BP compared to treatment with doxazosin and bisoprolol. Patients enrolled into the study were already being treated according to guidelines with maximally tolerated doses of a combination of three drugs: an angiotensin-converting-enzyme (ACE) inhibitor or angiotensin receptor blocker; a calcium channel blocker; and a thiazide-type diuretic.
The study randomized 335 patients with uncontrolled BP (seated clinic systolic BP ≥140 mmHg [or ≥135 mmHg for patients with diabetes] and a home systolic BP of ≥130 mmHg) to sequentially receive 12 weeks of treatment with spironolactone (25−50 mg), bisoprolol (5−10 mg), doxazosin (4−8 mg modified release) and placebo in random order. Some 14 patients were lost to follow-up.
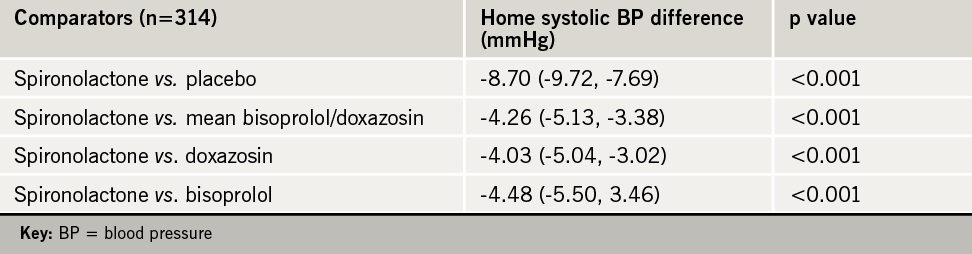
The primary end point was the average home systolic BP for each of the treatments, with clinic systolic BP being a secondary end point. The main results are shown in table 1. Spironolactone was well tolerated in the study with no significant excess adverse effects.
The findings “challenge the concept that resistant hypertension cannot be treated adequately with drug therapies, and suggest that treatments which have a natriuretic action, in that they promote sodium excretion, are likely to be the most effective,” said Professor Williams.
Discussant of the study, Professor Guiseppe Mancia (University of Milan, Italy) said very few studies have compared four treatment steps and the results were not only statistically significant but also clinically significant. He said he had no doubt that the result would have implications for future treatment and guidelines.
‘Win-win’ combination of diuretics
The combination of two commonly used diuretics – hydrochlorothiazide and amiloride − each at half dose, can significantly reduce BP without the side effects caused by full doses of either alone, according to results from the PATHWAY 3 study.
Announced at the same Hot Line session, Professor Morris Brown (University of Cambridge) said: “That matched doses of the two classes of diuretics could neutralise undesirable effects while potentiating the desirable is an important discovery”.
The use of thiazide-like diuretics has declined in recent years due to concerns that they increase the risk of diabetes, possibly due to potassium depletion. Potassium-sparing diuretics, such as amiloride, therefore offer a potential solution but require increased monitoring due to the theoretical risk of high potassium levels.

The study was carried out on 440 patients with uncontrolled hypertension, eligible for diuretic treatment, with a least one additional component of metabolic syndrome. They were randomised to receive either amiloride 10 mg alone, hydrochlorothiazide 25 mg alone, or a combination of both at half dose for 12 weeks, followed by another 12 weeks at double the dose for all groups.
Results showed the combination of amiloride and hydrochlorothiazide was a “win-win” said Professor Brown. Amiloride 10–20 mg had the opposite effects to hydrochlorothiazide (25–50 mg) on two-hour glucose and postassium (p< 0.01) but achieved the same fall in BP (-14 mmHg).
These results will mean the British Hypertension Society investigators will be recommending this combination, said Professor Brown.
In brief: hypertension
- Good news for ARNIs
There was more good news for the angiotensin receptor/neprilysis inhibitor (ARNI) LCZ 696 (sacubitril/valsartan) which was the highlight of last year’s congress when the PARADIGM study showed its efficacy in heart failure. This year, results from the PARAMETER study in elderly patients with hypertension, showed that LCZ696 could also significantly reduce central aortic systolic pressure and central aortic pulse pressure when compared to olmesartan.
Heart failure highlights
SERVE-HF: ASV devices cause harm in chronic heart failure
Use of an adaptive servo-ventilation (ASV) device to treat central sleep apnoea was shown to increase mortality in heart failure patients, according to the SERVE-HF (Treatment of Sleep-Disordered Breathing With Predominant Central Sleep Apnoea by Adaptive Servo Ventilation in Patients With Heart Failure) study.
The results were described as “practice-changing guidance for the treatment of chronic heart failure (CHF)” by co-principal Investigator Professor Martin Cowie (Imperial College, London), who presented the results in the Hot Line session. The study was also published simultaneously in the New England Journal of Medicine (doi: 10.1056/NEJMoa1506459).
The study randomly assigned 1,325 CHF patients with a reduced ejection fraction ≤45%, to either guideline-based medical management alone (control group) or with the addition of ASV for at least five hours per night for seven days a week.
After a median follow-up of 31 months, ASV effectively treated the sleep apnoea but had no effect on the primary end point: a combination of all-cause death, life-saving cardiovascular intervention or unplanned hospitalisations for worsening heart failure.
All-cause and cardiovascular mortality were higher in the ASV group than in the control group: 34.8% vs. 29.3% (HR 1.28; p=0.01) and 29.9% vs. 24% (HR 1.34; p=0.006), respectively.
Professor Cowie said the finding was a surprise and “disappointing”, adding that the investigators are “still trying to make sense of the data”. He speculated that Cheyne-Stokes respiration may be a compensatory mechanism and eliminating this may cause harm.
The investigators pointed out that the findings cannot be extrapolated to heart failure patients who have a preserved ejection fraction, or to those with predominantly obstructive sleep apnoea, or the use of continuous/automatic positive airway pressure devices (CPAP/APAP).
OPTILINK-HF: automated alerts did not improve outcome
Heart failure patients whose implantable cardioverter defibrillators (ICDs) include an automated alert when they have increased pulmonary congestion did not have improved outcomes compared to patients with regular ICDs, according to the OPTILINK-HF trial.
The trial enrolled 1,002 patients with ICDs and used telemonitoring to automatically transmit alerts to the patient’s doctor. Half of the patients were randomly assigned to have the automated transmission turned on and the others to have it turned off (standard care).
Presenting the results, Professor Michael Bõhm (Saarland University Hospital, Homburg, Germany) said that after 18 months follow-up, there was no significant difference between groups in the primary end point, a composite of all-cause death and cardiovascular hospitalisation.
During the study, 1,748 ‘fluid threshold crossings’ triggered an alert, with 76% of these being successfully transmitted from the patient’s ICD to the physician. Medical action was only taken in 30% of the successful transmissions as in the remaining cases the ‘fluid threshold crossing’ alert was not considered clinically actionable. Discussion of the findings suggested that ‘fluid threshold crossings’ were not the only important factor leading to decompensation and further clinical end points were needed to evaluate the system.
In brief: heart failure
- New MRA looks promising in ARTS-HF
Results of ARTS-HF (Mineralocorticoid Receptor Antagonist Study in Heart Failure trial) showed similar reductions in levels of the heart failure biomarker, NT-proBNP, between eplerenone and finerenone (a new highly selective non-steroidal mineralocorticoid antagonist ) in heart failure patients with type 2 diabetes and/or chronic kidney disease. The study enrolled 1,066 patients randomised to eplerenone (n=224) or five different finerenone doses (2.5–20 mg) for 90 days. Secondary end points, including all-cause and cardiovascular mortality were decreased with all but the lowest dose of finerenone. The incidence of adverse events and hyperkalaemia were similar in both groups.
- CUPID-2 shows ‘long and bumpy’ road for gene therapy

The largest study to date of gene transfer aimed at correcting an enzyme abnormality involved in myocardial contraction and relaxation, did not improve outcomes in heart failure patients with reduced ejection fractions. CUPID-2 enrolled 250 patients from 67 centres who were randomised to one dose of an intracoronary infusion of the SERCA2a gene using an adeno-associated virus vector, or to placebo, and followed for at least one year. There were no improvements in the primary end point − hospitalisations or ambulatory treatment for worsening heart failure − or a number of secondary end points in either group. No safety issues were noted and commentators suggested that, while we need to work out why encouraging animal studies did not translate into clinical benefit, this road is “long and bumpy” and it would be a scientific mistake to abandon gene therapy in heart failure.
- Aspirin therapy may cause harm in heart failure
Researchers from Denmark conducted a nationwide study of patients with heart failure and sinus rhythm in order to assess the safety and efficacy of aspirin therapy. The study found it offered no benefit in this cohort of patients, regardless of the type of heart failure. Aspirin therapy was, however, associated with an increased risk of bleeding in patients with ischaemic heart disease. Commenting on the Danish study, Professor Theresa McDonagh, (King’s College Hospital, London) said that while the study showed the possible harm of aspirin in heart failure treatment, she did not question the use of aspirin in treating heart attacks. She called for proper clinical trials to assess the efficacy of aspirin in patients who have heart failure following a heart attack.
Acute MI highlights
CIRCUS: no advantage with cyclosporine in STEMI
The CIRCUS (Cyclosporine to Improve Clinical Outcome in ST-elevation Myocardial Infarction) trial showed no advantage in the off-label clinical efficacy of cyclosporine in acute ST-elevation myocardial infarction (STEMI).
The study, presented by Professor Michel Ovize (Clinical Investigation Centre of Lyon, France), included patients with anterior STEMI undergoing primary percutaneous coronary intervention (PCI), who were randomised before the procedure to either a single IV bolus of 2.5 mg/kg of cyclosporine (n=475) or placebo (n=495). The hypothesis was that the drug might attenuate reperfusion injury and reduce infarct size.
At one year, the primary end point − the composite of all-cause mortality, rehospitalisation for heart failure, worsening heart failure during initial hospitalisation, and left ventricular adverse remodelling − occurred in 59.0% and 58.1% of the cyclosporine and control groups, respectively (p=0.77). There was no difference in the components of the primary end point between the groups.
The paper was published simultaneously in the New England Journal of Medicine (doi: 10.1056/NEJMoa1505489). In an accompanying editorial, Dr Derek Hausenloy and Professor Derek Yellon (University College, London) put forward some explanations for the negative findings. They suggested that the evidence supporting the cardioprotective effects of cyclosporine are limited. Also some of the end points and the cyclosporine formulation may not have been suitable.
Peri-infarct pacing improves outcomes in large MIs
Peri-infarct pacing does not improve outcomes in patients who have had a large myocardial infarction, according to the PRomPT study.
Presented by Dr Greg Stone (New York Presbyterian Hospital, New York, USA), the PromPT (Pacing Remodeling Prevention Therapy: Peri-infarct Zone Pacing to Prevent Adverse Left Ventricular Remodeling in Patients with very Large Myocardial Infarction) study investigated the effect of early activation of the heart’s peri-infarct region with left ventricular pacing on left ventricular remodelling.
This prospective, multi-centre, controlled study enrolled 126 patients with a large, first STEMI and a QRS duration <120 ms within 10 days of symptom onset, and randomised them to left ventricular (LV) and right ventricular (RV) pacing, LV pacing only, or no pacing (control group). Those randomised to pacing received cardiac resynchronisation therapy plus defibrillator therapy (CRT-D) with left ventricular and right ventricular leads implanted in a peri-infarct region, as determined by echocardiography.
Patients were followed-up for 18 months. When the two pacing groups were pooled together and compared with the control group, the primary end point − change in left ventricular end-diastolic volume − was similar (16.7 ± 30.5 ml in the pacing groups vs. 15.3 ± 28.6 ml in the control group, p=0.92). The rates of death and hospitalisation for heart failure were also similar between the groups, and there were no differences in quality of life and New York Heart Association class between the groups.
Dr Stone commented that “the results of this trial are sufficiently neutral such that future studies will most likely not explore peri-infarct LV pacing to improve outcomes for patients with large MIs”. Study discussant Dr Frank Ruschitzka (University Hospital, Zurich, Switzerland), suggested that we might “need such crazy ideas”!
The trial was published simultaneously in the European Heart Journal (doi: 10.1093/eurheartj/ehv436).

In brief: acute MI
- ALBATROSS shows no benefit of MRAs in MI without HF
The addition of mineralocortoid receptor antagonists (MRAs) to standard therapy showed no benefit in myocardial infarction (MI) patients without heart failure (HF). ALBATROSS investigated the benefit of six months of aldosterone blockade with spironolactone early after acute MI onset on top of standard therapy in 1,600 patients. The primary end point − a composite of death, resuscitated cardiac arrest, significant ventricular arrhythmia, class IA indication for ICD and new or worsening heart failure − were similar between the groups (p=0.81). Mortality was also similar (p=0.26) but this was lower in patients with STEMI randomised to spironolactone compared to placebo; and tended to be higher in patients with non-ST-elevation MI (NSTEMI).
- New rapid assay enables quicker triage
Patients arriving at the emergency department with chest pain suggestive of acute myocardial infarction (AMI) can be triaged more quickly and more safely using a new rapid high sensitivity troponin I assay with refined cut-offs, the German BACC study suggests. This confirms the recent update of ESC NSTEMI guidelines.
CAD highlights
18-month follow-up in heterozygous FH from ODYSSEY
With both proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibodies, alirocumab and evolocumab, now approved in Europe and the USA, presentations at the Congress were focused on consolidation of safety and efficacy data.
A pooled analysis of ODYSSEY data in 1,257 patients with heterozygous familial hypercholesterolaemia (FH) was one of the highlights in a Clinical Trial Update session. This comprised the largest dataset of FH patients treated with a PCSK9 inhibitor followed for up to 78 weeks treatment with alirocumab, on top of a maximally tolerated statin ± other lipid-lowering therapy.
Data were pooled separately for ODYSSEY FH I and II studies (mean low-density lipoprotein cholesterol [LDL-C] 3.66 mmol/L, n=735), and for the ODYSSEY HIGH FH study plus FH patients included in ODYSSEY LONG TERM (mean LDL-C 4.36 mmol/L, n=522). In each study, diagnosis of heterozygous FH was made either by genotyping or using clinical criteria (Simon Broome criteria or World Health Organisation/Dutch Lipid Network criteria with a score of >8 points).
Alirocumab was administered at a dose of 75 mg/titrating to 150 mg every two weeks, depending on LDL-C response at week 8, in ODYSSEY FH I and II, and as 150 mg every two weeks in the other two studies.
At week 24, both pooled analyses showed more than 55% reduction in LDL-C from baseline versus placebo (placebo corrected change: -56.1% in ODYSSEY FH I and II, and -57.1% in ODYSSEY HIGH FH/LONG TERM). There was no evidence of tolerance to alirocumab, as the LDL-C lowering response was sustained over 78 weeks (-56.1% and -63.2%, respectively). Most importantly, more than 75% of patients in ODYSSEY FH I and II attained LDL-C goal <2.6 mmol/L and 63% attained an LDL-C goal <1.8 mmol/L. Response data were slightly less in the other analysis (65% and 56%, respectively), reflecting the more severe phenotype of patients enrolled in ODYSSEY HIGH FH.

The adverse event profile of alirocumab was generally similar to that reported for placebo, with no increase in the incidence of myalgia (5.1% vs. 6.2%). Previously, very few heterozygous FH patients would attain LDL-C goal even with statin and additional lipid-lowering therapy. These findings from ODYSSEY clearly show the potential of PCSK9 inhibition in this high cardiovascular risk patient population.
According to Professor John Kastelein (Academic Medical Center, Amsterdam, the Netherlands): ‘Treatment with alirocumab lowered LDL-C to levels that were unobtainable with existing currently standard-of-care therapy in patients with heterozygous FH. Importantly, the LDL-C lowering response was also sustained in patients with severe FH who had markedly elevated LDL-C levels at baseline. Treatment was also well tolerated in the long-term.”
In brief: CAD
- The importance of LDL-C not diminished in statin era
LDL-C is acknowledged as one of key drivers of coronary heart disease (CHD) risk. This holds true even in the statin era, according to a report from Duke Clinical Research Institute, Durham, USA. Investigators compared the association between LDL-C and new onset CHD in the pre-statin (1983–1996) and post-statin (1997–2007) eras, based on data from more than 4,000 adults in the Cardiovascular Health Study and the Framingham Offspring Study. Not surprisingly, use of statins increased from 7.3% in 1997 to 30.7% in 2007, and correspondingly, the CHD event rate decreased from 1.35 to 1.20 per 100 patient-years of follow up. Yet, although mean baseline LDL-C in CHD cases was lower in the post-statin cohort (140 vs. 132 mg/dL [3.62 vs. 3.41 mmol/L], p=0.001), the association between elevated LDL-C and CHD risk did not differ significantly (p=0.89).
- Unmet clinical needs in high risk patients
Findings from DYSIS (Dyslipidaemia International Study) II clearly highlight the unmet clinical needs, in terms of LDL-C goal attainment, in high cardiovascular risk patients. The global analysis included 3,867 patients with acute coronary syndrome (ACS) and 6,794 with stable CHD. In the ACS subgroup, management of elevated LDL-C was still far from optimal, with only 20−25% of patients attaining recommended LDL-C goal; goal attainment was even worse in the Middle East region. Failure to implement appropriate high-intensity statin, as well as consider additional lipid-lowering therapy were key obstacles. In another presentation based on a cohort of 29,565 very high cardiovascular risk patients included in the Cegedim General Practice Database (France), slightly more than half (58%) were treated with a statin, with only 13% on high-intensity statin therapy. Less than one in five patients (16%) attained the recommended LDL-C goal of <1.8 mmol/L. Much remains to be done to improve clinical management of high-risk patients.
- More news with the PCSK9 inhibitors
A pooled analysis of more than 3,000 patients included in the PROFICIO clinical trial programme showed that the LDL-C lowering response to evolocumab (140 mg two-weekly or 420 mg monthly) was similar irrespective of age, gender, glycaemic status or cardiovascular risk. Additionally, there were safety data from 382 statin intolerant patients enrolled in GAUSS and GAUSS-2, who were subsequently randomised to evolocumab or standard of care in OSLER. Over a median 11 month follow-up, there was no indication of any difference in susceptibility to muscle-related symptoms between the two groups; myalgia was reported by 8.4% of patients in each group, and myositis (muscle symptoms with elevation in creatine kinase) reported by 14.7% in the evolocumab group versus 14.5% with standard of care.
Elevated lipoprotein(a) [Lp(a)] is now established as causal for cardiovascular disease. However, with the withdrawal of niacin (nicotinic acid) subsequent to HPS2-THRIVE, there is a lack of efficacious treatments. PCSK9 monoclonal antibody therapy has been shown to reduce Lp(a). In the largest pooled analysis to date, including 4,915 patients in the ODYSSEY clinical trial programme, with or without statin, alirocumab reduced Lp(a) by 23–29%. Importantly, this response was durable and sustained for up to 78 weeks. Thus, not only does PCSK9 inhibition effectively lower LDL-C in high cardiovascular risk patients, but there is incremental benefit in lowering Lp(a).
- What’s next for PCSK9 inhibition?
Last, but certainly not least, there were exciting data presented for a novel strategy to target PCSK9, aimed at silencing the PCSK9 messenger RNA (small molecule RNA interference therapeutics). In a Phase I study in hypercholesterolaemic patients, some on statins, a single subcutaneous injection of ALN PCSsc (500 mg) reduced LDL-C by up to 78% (mean maximal reduction 58% over the dose range 25–800 mg). This LDL-C lowering response was comparable with that reported with PCSK9 monoclonal antibody therapy. However, a key finding was that the response to a single injection of ALN PCSsc was sustained for at least 140 days, suggesting future potential for dosing every three months or less. Phase II trials with this agent are due to commence in Europe late 2015.
AF and pacing highlights
Stroke prevention in the real world: balancing efficacy and safety
Much of the reportage in the atrial fibrillation (AF) arena was focused on real-world use of oral anticoagulation to prevent stroke.
From the GARFIELD-AF Registry, it is evident that the UK still lags behind other European countries in the use of non-vitamin K antagonist oral anticoagulants (NOACs) in patients with newly diagnosed non-valvular AF (n=17,475). At 24 months from the date of commercial introduction, less than 5% of UK patients were prescribed a NOAC compared with almost 50% in France, although reimbursement is likely to be a factor.
Moreover, while there were no gender differences among AF patients prescribed NOACs, one-year outcomes data (n=28,624) from GARFIELD-AF over the period June 2010−March 2014 showed that women appear to be at greater risk of stroke and bleeding complications compared with men. The reasons for this are not yet fully understood, although longevity, use of hormone replacement therapy and suboptimal management may all play a part. Professor Barbara Casadei (British Heart Foundation Professor of Cardiovascular Medicine, University of Oxford) discusses why women with cardiovascular disease may be less optimally managed in this podcast.
One of the key concerns for the use of NOACs in the real-world is the risk of bleeding complications. This issue was the focus of a number of presentations. In XANTUS in 6,784 unselected patients with non-valvular AF from everyday practice (mean CHA2DS2-VASc score 3.4, CHADS2 score 2.0, 19% with previous stroke), 96.1% did not experience treatment-emergent all-cause death, major bleeding or stroke with rivaroxaban treatment. Major bleeding occurred in 128 (1.9%) patients with intracranial bleeding in 26 cases (seven fatal). Major bleeding, all-cause death, and thromboembolic events were more prevalent at the lower rivaroxaban dose (15 mg versus 20 mg once daily). However, it should be borne in mind that outcomes were not adjusted for baseline risk factors.
A key question is whether the NOACs have similar bleeding risk. Three abstracts presented at the meeting addressed this issue by looking at retrospective analyses of US real-world data, based on more than 125,000 non-valvular AF patients newly initiated on a NOAC or switched from warfarin (see table 2). As shown by Lip et al., the cumulative incidence of major bleeding was significantly lower with apixaban compared with rivaroxaban or warfarin, with no statistically significant difference between apixaban and dabigatran. Similarly, Lin et al. showed that apixaban conferred a significantly lower risk of any bleeding than rivaroxaban or warfarin, but did not differ from dabigatran over the first six months after initiating treatment; in this analysis, most patients were on the recommended standard dose (see table 2).
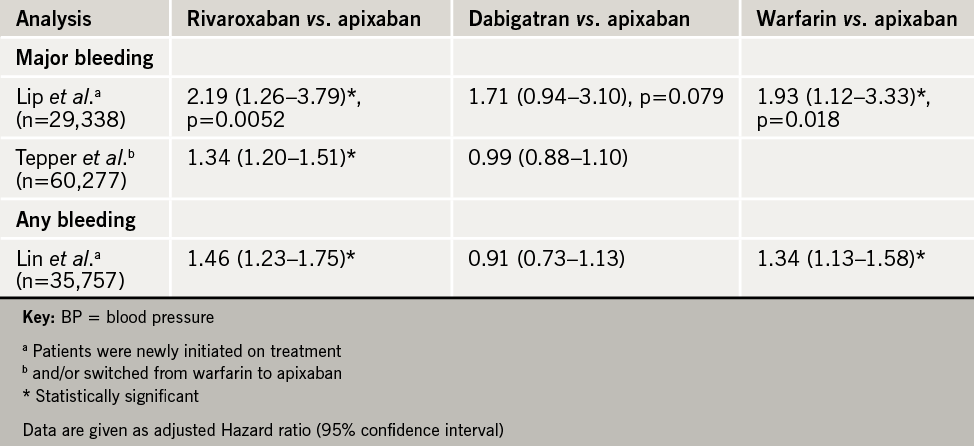

These findings have important implications for hospitalisation and resource use. Lower risk of a major bleeding event with apixaban vs. rivaroxaban in newly initiated AF patients translated to reduction in all-cause hospitalisation (adjusted hazard ratio, rivaroxaban vs. apixaban 1.6, 95% CI 1.3–1.9; dabigatran vs. apixaban 1.4, 95% CI: 1.1–1.7), as well as lower healthcare costs by more than US $4,000 per patient (USA data). While there are obvious limitations in extrapolation to the UK, these findings are undoubtedly pertinent for UK clinical decision making, given that the NHS faces increasing financial restraint.
In brief: AF and pacing
- LEADLESS II: potential for leadless cardiac pacemaker?
In this prospective, non-randomised phase II study (n=526, mean age 75.8 years), nonsurgical implantation of this single-chamber rate-adaptive pacemaker showed good safety and reliability function. At six months, 90% of patients met the primary effectiveness end point (clinically acceptable capture thresholds ≤2.0 V at 0.4 ms, and sensing [R-wave ≥5.0 mV or ≥implant value]). In addition, 93.3% of patients were free from serious adverse device effects (SADEs). Of those who did experience such events, there was a low incidence of cardiac perforation (1.3%), device dislodgement with successful percutaneous retrieval (1.7%), and capture threshold elevation requiring percutaneous retrieval and placement of a new leadless pacemaker (1.3%). Increased operator experience would be expected to improve the SADE rate.
- Antiarrhythmic therapy versus ablation?
In the EAST-AF (Efficacy of Antiarrhythmic Drugs Short-Term Use after Catheter Ablation for Atrial Fibrillation) study (n=2,044, mean age 63 years), addition of antiarrhythmic drugs (AAD) for 90 days after ablation did not impact late AF recurrence. Although there was a temporary benefit of AAD, with a significantly lower rate of early arrhythmia recurrences at 90 days (59.0% vs. 52.1%, p=0.01), this effect did not persist after discontinuation of treatment, with no significant impact on one-year clinical outcomes. These findings confirm the results of other trials such as AMIO-CAT (AMIOdarone after CATheter ablation for AF).
MANTRA-PAF (Medical Antiarrhythmic Treatment or Radiofrequency Ablation in Paroxysmal Atrial Fibrillation) previously showed no significant difference at two years between first-line treatment with AAD or ablation with respect to the burden of AF in Paroxysmal Atrial Fibrillation (PAF). However, five-year follow-up data (n=245) reported showed that outcome was significantly better with ablation, both in terms of any AF (p=0.003) and symptomatic AF (0=0.02). Importantly, improvement in quality of life reported at two years was sustained after five years with no differences between the two groups. Overall, these data suggest that an active rhythm control strategy is feasible and may prevent progression from paroxysmal to persistent types of AF, although it should be acknowledged that ablation techniques were not comparable with 2015 standards. Additionally, the risk of severe complications with ablation needs to be taken into account in routine practice.
- BELIEF in left atrial appendage?
Findings from the BELIEF trial support the notion that left atrial appendage (LAA) is a relevant, under-reported trigger for AF. The study included 173 patients with long-standing AF (defined as persisting beyond one year), who were randomly assigned to standard treatment alone (pulmonary vein isolation and ablation) or standard treatment plus LAA ablation. Additional LAA ablation HR 1.92, p=0.001). Success rates at two years were also significantly better in the LAA group versus standard treatment (76% vs. 56%, p=0.003). Complication rates did not differ between the two groups. The trial findings indicate LAA isolation is feasible and confers added benefit, in terms of lower recurrence of AF, compared with standard treatment alone. However, the belief in LAA is not shared by all experts. Given methodological reservations, further studies are needed before LAA can be recommended as an integral part in the management of catheter ablation of longstanding persistent AF.
Diabetes and pharmacology highlights
News from IMPROVE-IT
Diabetes patients obtained greater benefit from ezetimibe plus simvastatin than those without diabetes, according to a report presented by Dr Robert P Giugliano (Brigham and Women’s Hospital, Boston, MA, USA). This was despite evidence that the diabetes subgroup (n=4,933) tended to be at higher risk, given that they were older, more likely to be female, hypertensive with a higher body mass index, and more often had prior myocardial infarction and coronary revascularisation.
Placebo-adjusted LDL-C levels were reduced to a greater extent with simvastatin-ezetimibe in diabetes patients (by 0.43 mmol/L vs. 0.37 mmol/L in patients without diabetes). This conferred a 5.5% absolute risk reduction in the primary end point (cardiovascular death, hospital admission for unstable angina, MI, coronary revascularisation day 30 or later, or stroke, HR 0.86; 95% CI 0.78–0.94, p=0.023) but not in non-diabetics (HR 0.98; 95% CI 0.91–1.04). However, Dr Giugliano stressed that clinicians should not withhold add-on ezetimibe in non-diabetics, underlining the heterogeneity of this high-risk patient group.
In another report from IMPROVE-IT, Dr Michael Blazing (Duke University Medical Center, Durham, USA) showed that the combination of ezetimibe plus simvastatin did not increase new-onset diabetes. This analysis defined new-onset diabetes by two criteria: initiation of diabetes medication or two consecutive fasting glucose values ≥7 mmol/L. Patients with pre-existing diabetes were excluded from the analysis. Overall, 1,414 (13.3%) patients developed new-onset diabetes, with similar numbers in each treatment group (720 with ezetimibe-simvastatin vs. 694 with simvastatin, HR 1.04, p=0.46). While the definition of new-onset diabetes used has limitations, in that haemoglobin A1c or glucose was not monitored, the findings support a lack of increase in risk of new-onset diabetes with ezetimibe.
Finally, there was reassurance in safety analyses that ezetimibe did not increase the frequency of muscle symptoms when added to simvastatin (19.6% vs. 19.0%, p=0.263). There was also no increase in cancer rates with this combination (hazard ratio 1.03, 95% CI 0.93–1.14, p=0.57).
In brief: diabetes and pharmacology
- Cardiovascular safety of new diabetes agents
A report from ELIXA (Evaluation of Lixisenatide in Acute Coronary Syndrome) shows that lixisenatide, a glucagon-like peptide-1 receptor agonist, did not significantly affect the rate of cardiovascular events in type 2 diabetes patients with ACS compared with placebo (13.4% vs. 13.2%, HR 1.02, 95% CI 0.89–1.17). These are the first data on the cardiovascular safety of this type of agent.
Findings from TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin) show similar rates for hospitalisation for heart failure over 2.9 years with sitagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, or placebo (HR 1.02, 95% CI 0.83–1.26, p=0.82 in multivariable analysis). These findings contrast with results from the SAVOR TIMI 53 and EXAMINE trials, possibly due to differences in the characteristics of the patient populations, background care, heart failure definition or intrinsic differences between the DPP-4 agents.
Five new ESC guidelines published
 Five new ESC guidelines were launched at the Congress for:
Five new ESC guidelines were launched at the Congress for:
- Infective endocarditis
- Management of ventricular arrhythmias and prevention of sudden cardiac death
- Non ST-elevation acute coronary syndrome
- Pericardial diseases
- Pulmonary hypertension (joint ESC/European Respiratory Society guidance)
Further information on all the guidelines can be found at http://www.escardio.org/
In brief: other highlights
ESC calls for legislation for trans fats
The ESC has called for regulatory intervention to reduce the content of industrial trans fatty acids (TFAs) in food. Last year the ESC released a position paper describing the detrimental effects of TFAs on heart health and mortality as ‘now beyond dispute’. An update given by Dr Steen Spender (Copenhagen University Hospital, Denmark) showed that individuals in countries, which rely on voluntary efforts to reduce TFAs in food can still access foods with high TFAs. He carried out a supermarket sweep in 20 European countries and found 312 of 600 foods had more than 2% of their content as TFAs. This exceeds the legislative limit now imposed in Denmark and Austria.
…and for air pollution
Three years ago the World Health Organization (WHO) estimated that one in eight of global deaths (around 7,000,000 each year) are attributable to air pollution, with the nanoparticles (≤2.5 µg) found in diesel and petrol exhaust, and nitrogen dioxide, being the main culprits. A Belgian study presented at the meeting looking at registry data for ST-elevation myocardial infarction over four years, found that particulate and nitrogen dioxide were associated with a 2.8 % and 5.1% increased risk in STEMI, respectively. EU limits for air pollution are currently less stringent than WHO standards. Is it time for cardiologists and health care professionals to call for change to policy, says the ESC.
Primary prevention needs new strategies
Better strategies are needed to manage and control risk factors. The secondary prevention arm of the latest EUROASPIRE IV study shows that the majority of patients in Europe are failing to meet their lifestyle, risk factor and therapeutic targets. The primary prevention arm in high-risk patients showed similarly disappointing results with no change found in the prevalence of smoking or obesity (including central obesity); and no improvement in the therapeutic control of BP, cholesterol or diabetes.
Holidays are good for the heart?
The benefits of the Mediterranean diet were extolled at the meeting as still being the gold standard. While, naps taken at midday are directly associated with reduced BP and prescription of few antihypertensive medications according to Dr Manolis Kallistratos (Asklepieion Voula General Hospital, Athens, Greece).
But beware those strong after-dinner coffees. According to an Italian study, coffee is associated with an increased risk of cardiovascular events in young adults with mild untreated hypertension. Investigators found a linear relationship between coffee and the risk of hypertension needing treatment. In heavy drinkers, the association reached statistical significance.
Electrical faults suggested for sudden cardiac death in young
A retrospective study from St George’s Hospital, London, on sudden cardiac death in young people (18–40 years) who engaged in regular sport during life found that the most common cause of death was sudden arrhythmic death syndrome and that 61% of these deaths occurred during exercise with almost 40% after exertion or at rest. Deaths were much higher in men than women, and in those with the congenital condition of arrhythmogenic right ventricular cardiomyopathy.
