Atrial fibrillation (AF) is one of the most common arrhythmias, affecting approximately 2% of the general population. Identifying AF after an ischaemic stroke is particularly important as it changes the recommended antithrombotic therapy from antiplatelets to anticoagulation. Currently, there is no clear consensus with regards to the duration of rhythm monitoring post-stroke. In our study, we aim to review some of the pivotal studies regarding rhythm monitoring after an ischaemic stroke and identify the percentage of patients who get referred for prolonged rhythm monitoring after a stroke by providing real-world data from the Ipswich hospital. To our surprise, we did not identify any patients who got referred for prolonged rhythm monitoring (ILR) and the proportion of patients who did not have a 24-hour tape was unexpectedly high. In addition, there was a clear tendency for patients with lacunar strokes not to get investigated with 24-hour tape.
Introduction
Atrial fibrillation (AF) is a very common cardiac arrhythmia affecting approximately 2% of the general population.1 Thromboembolic events and, in particular, stroke is one of its most fearsome complications and in the absence of appropriate anticoagulation, AF increases the risk of stroke five-fold.1 In addition, strokes related to AF have increased mortality and morbidity, while their survivors are at increased risk of recurrent stroke.2 Anticoagulation with dose-adjusted warfarin or direct oral anticoagulants significantly reduces the risk of stroke by almost 70%; making consideration of anticoagulation an imperative aspect of stroke management when AF has been identified.3 In the absence of identified AF, patients with ischaemic stroke should be treated with appropriate antiplatelet agents.
In post-stroke patients, the challenge lies in the paroxysmal and frequently silent nature of AF, which makes detection difficult. Therefore, monitoring is frequently necessary in order to detect silent, paroxysmal AF and instigate appropriate management. Currently, there is no clear consensus regarding the duration of monitoring of patients with stroke without identified AF on initial assessment. US guidelines (2014) suggest prolonged rhythm monitoring (about 30 days) within six months of the index event, while the European Society of Cardiology (ESC) guidelines (2016) suggest short-term electrocardiogram (ECG) recording followed by continuous monitoring for at least 72 hours.4,5 In our study, we aim to review some of the pivotal studies regarding rhythm monitoring after an ischaemic stroke and identify what percentage of patients get referred for prolonged monitoring by providing some real-world data from the Ipswich hospital, UK.
Prolonged rhythm monitoring after ischaemic stroke
Ischaemic stroke is a heterogeneous condition with various mechanisms (large-vessel atherosclerosis, small-vessel ischaemia, cardioembolic, etc.) and risk factors (hypertension, diabetes, smoking, etc.). Irrespective of our certainty with regards to the mechanism of a particular stroke, the main focus in stroke management should be identifying and modifying stroke risk factors. Identifying AF in patients with an ischaemic stroke does not imply explicit causality with regards to the mechanism of the stroke, but is particularly important as it changes the recommended antithrombotic therapy from antiplatelet drugs to oral anticoagulation, which is significantly more effective.6
Two prospective, randomised clinical trials over the last two years have confirmed the logically expected finding that prolonged rhythm monitoring is superior to standard monitoring for the detection of AF after a seemingly cryptogenic stroke. In the EMBRACE (30-Day Cardiac Event Monitor Belt for Recording Atrial Fibrillation after a Cerebral Ischemic Event) trial,7 572 patients with cryptogenic ischaemic stroke or transient ischaemic attack (TIA) within the previous six months, were randomised to undergo a 30-day event-triggered recorder (intervention group) or an additional 24-hour monitoring (control group). All patients were older than 55 years, without known AF and were diagnosed with cryptogenic stroke after 12-lead ECG, 24-hour Holter monitoring, brain and neurovascular imaging and echocardiography. AF lasting more than 30 seconds was detected in 16.1% in the intervention group as compared with 3.2% in the control group (95% confidence interval [CI] 8–17.6; p<0.001). There was an incremental yield of prolonged ECG monitoring for the detection of AF throughout the monitoring period. AF lasting at least 2.5 minutes was detected in 9.9% in the intervention group as compared with 2.5% in the control group (95% CI 3.4–11.3; p<0.001). Also, by 90 days, 18.6% of the intervention group had been prescribed oral anticoagulants as compared with 11.1% in the control group (95% CI 1.6–13.3; p=0.01).7 Further analysis of the EMBRACE trial showed that the probability of detecting AF increased depending on the number of premature atrial ectopics per 24 hours.8
In the CRYSTAL AF (Cryptogenic Stroke and underlying Atrial Fibrillation) trial,9 441 patients with cryptogenic stroke or TIA were randomised within 90 days of the index event to receive an implantable cardiac monitor (ICM) or conventional follow-up (scheduled and unscheduled visits with ECG monitoring performed at the discretion of the site investigator). Patients were older than 40 years of age and were diagnosed with cryptogenic stroke after 12-lead ECG, 24-hour monitor, transoesophageal echocardiogram, thrombophilia screen if <55 years of age and magnetic resonance angiography (MRA), computed tomography angiography (CTA) or catheter angiography of head and neck. By six months, AF had been detected in 8.9% of patients in the ICM group compared with 1.4% of patients in the control group (95% CI 1.9–21.7; p<0.001). By 12 months, AF had been detected in 12.4% of patients in the ICM group versus 2% of patients in the control group (95% CI 2.6–20.8; p<0.001). Treatment modifications were not mandated by the trial protocol, but the majority of patients (97%) with detected AF received oral anticoagulation, confirming a change in management plan. At six months, 10.1% of patients in the ICM group were receiving anticoagulation versus 4.6% in the control group (p=0.04) and 14.7% versus 6% at 12 months (p=0.007). In the ICM group, most of the episodes of AF were asymptomatic (74% at six months and 79% at 12 months).9 Further analysis of the CRYSTAL AF trial found that increasing age and prolonged PR were associated with increasing AF incidence. In contrast to the EMBRACE trial, premature atrial ectopics were not found to be significant predictors of AF.10
More recently, the STROKESTOP study (not conducted in the context of recent stroke) has demonstrated that ambulatory patient-triggered ECG can increase the detection of AF.11 In the STROKESTOP study, 75/76-year-olds were screened with ambulatory intermittent patient-triggered ECGs over a two-week period: 3% were found to have previously unknown AF and more than 90% of them were initiated on oral anticoagulation. Of the participants with known AF, 2.1% were not on oral anticoagulation making up a total of 5.1% of untreated AF in the screened population. Their multi-variable analysis showed that the strongest predictor of AF (new or known) was heart failure followed by stroke/TIA and diabetes. This study demonstrates the significance of screening for AF even in the general population.11
Materials and methods
All patients with a confirmed ischaemic stroke or diagnosis of TIA at the Ipswich hospital between 1 June 2015 and 31 August 2015 were identified. We identified 101 patients admitted with a stroke and 71 patients diagnosed with TIA. The medical records including the hospital admission records, follow-up records and investigations were reviewed.
Results
Table 1 shows the patient characteristics of the 101 patients admitted with a stroke and 71 patients diagnosed with TIA when assessed in TIA clinic. Both groups of patients had similar characteristics with respect to age and common risk factors for strokes.

It was clear from reviewing the medical notes that the stroke physicians appropriately tried to tailor the investigations according to each particular patient. There were 48 patients admitted with a stroke and 56 patients diagnosed with TIA who had ultrasound (US) of the carotids. If there was a concern or uncertainty about the findings of the carotids US, then the patient was investigated further with MRA of the carotids.
Figure 1 shows the investigations that the patients admitted with a stroke underwent. All 101 patients admitted with stroke had an ECG on admission: 71 (70.2%) patients were in sinus rhythm while 24 (23.7%) were in AF/flutter (old) and six (5.9%) had newly identified AF/flutter on admission ECG. In 53 out of 71 patients in sinus rhythm on admission, identifying AF/flutter would lead to change in management. We excluded patients who were anticoagulated for another reason, or patients who had a catastrophic stroke and either died during their hospital stay or were deemed candidates for palliative care only. Out of those 53 patients, one declined further investigations, 13 (24.5%) did not have 24-hour tape while 39 (73.5%) had telemetry or 24-hour tape or a pacemaker check. Out of 39 patients, seven (17.9%) had newly diagnosed AF/flutter, while one patient out of three that had an R-test (five-day monitoring) had newly diagnosed AF/flutter as well.
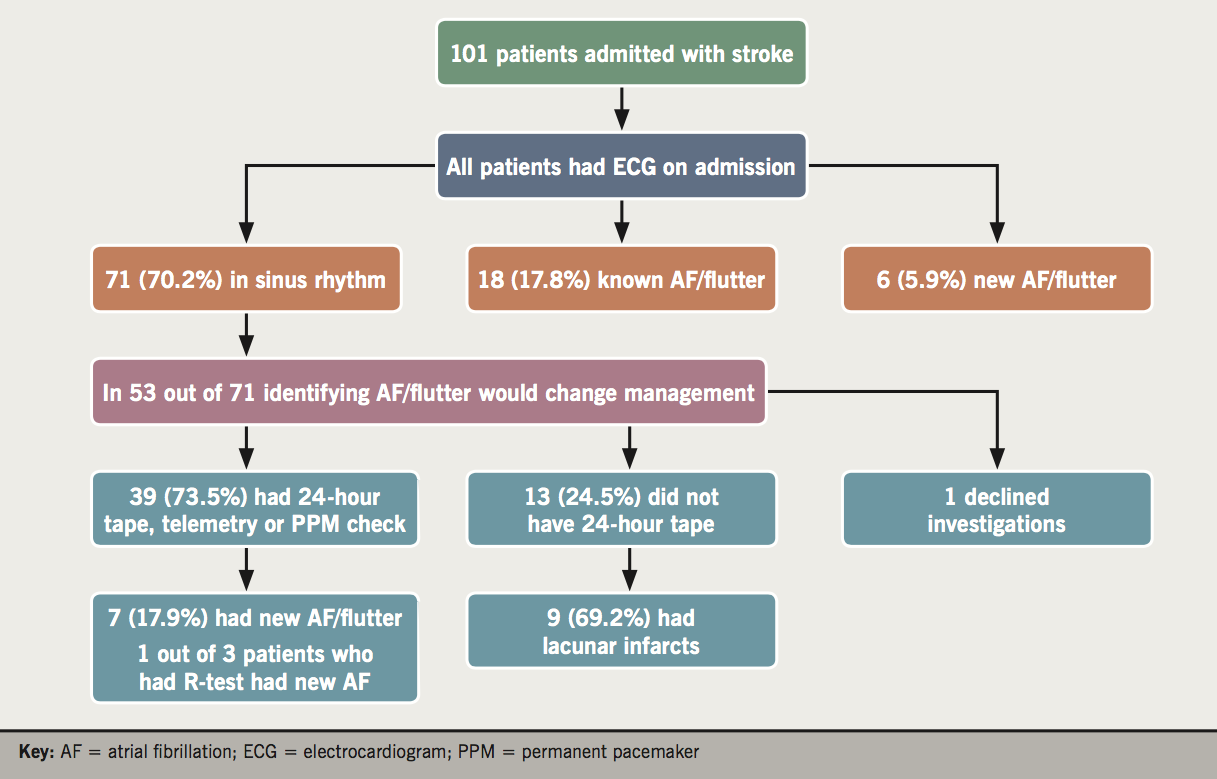
It is evident that a significant proportion of patients (13 out of 51, 24.5%) did not undergo a 24-hour tape. It also became apparent that a significant proportion of these patients (nine out of 13, 69.2%) had lacunar strokes. Therefore, we decided to analyse further the stroke patients and compare the two subgroups of stroke patients (non-lacunar and lacunar strokes). Table 2 shows the patient characteristics of patients with non-lacunar and lacunar strokes. It demonstrates that these two subgroups share similar patient characteristics and risk factors for stroke. Figure 2 shows the investigations of patients admitted with non-lacunar and lacunar strokes. All 34 patients with lacunar strokes had an ECG on admission: 25 patients (73.5%) were in sinus rhythm, while six (17.6%) were in AF/flutter (old) and three (8.8%) had newly diagnosed AF/flutter on the admission ECG. There were 17 (50%) patients who underwent telemetry or 24-hour tape or a pacemaker check. Out of those 17 patients, three (17.6%) had AF (old) and two (11.6%) had newly identified AF.

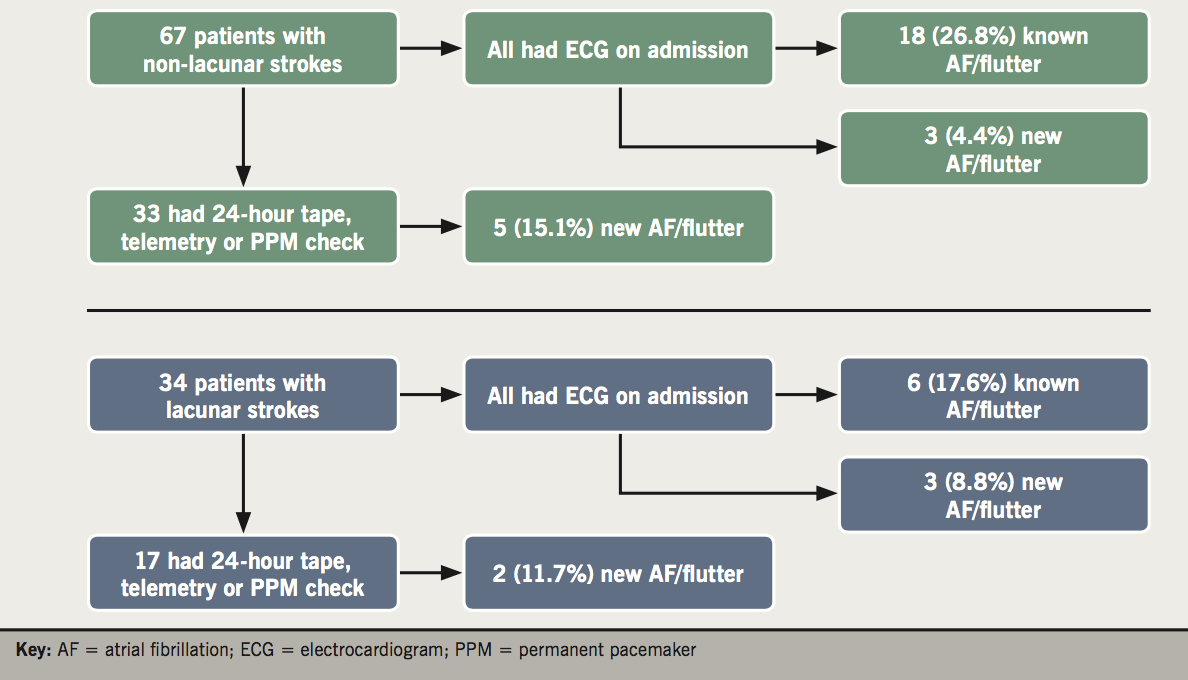
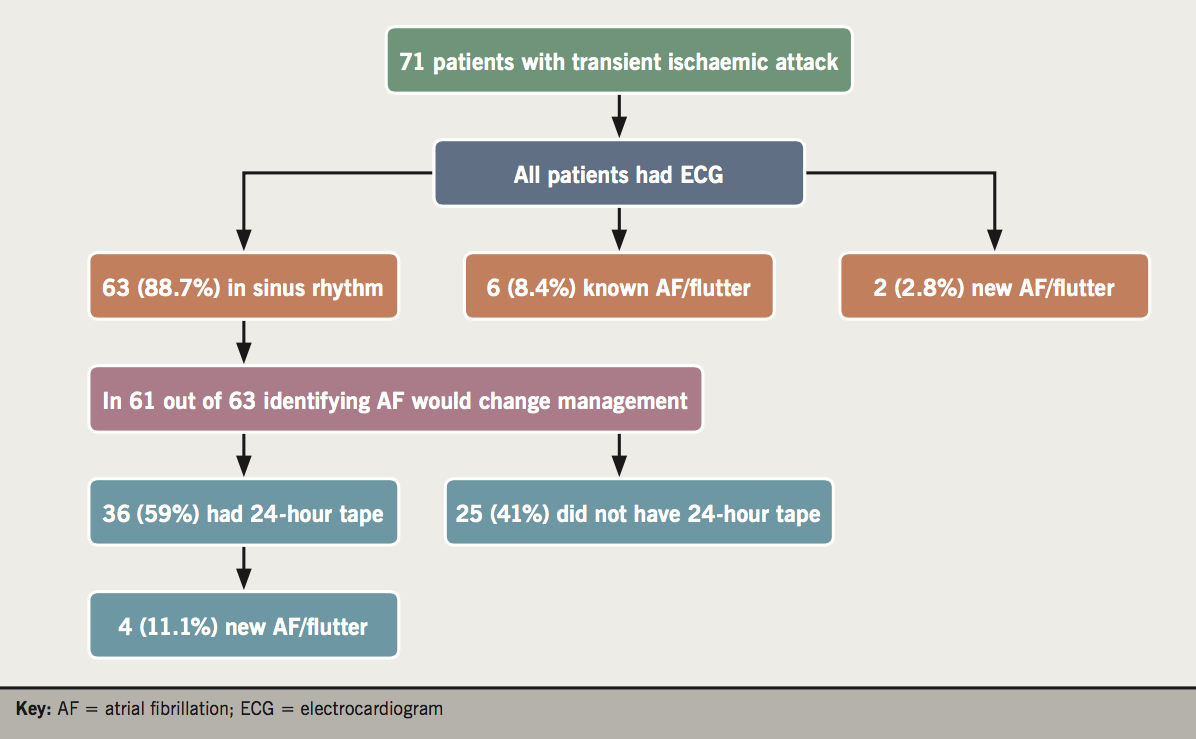
Figure 3 shows the investigations the patients diagnosed with TIA in the TIA clinic underwent. All 71 patients had an ECG: 63 patients (88.7%) were in sinus rhythm, while six (9.5%) were in AF/flutter (old) and two (2.8%) had newly identified AF/flutter. In 61 out of 63 patients in sinus rhythm, identifying AF/flutter would change management. We excluded two patients who were anticoagulated for another reason. Out of those 61 patients, 25 (41%) did not undergo a 24-hour tape while 36 (59%) had a 24-hour tape. Four out of 36 patients (11.1%) that had a 24-hour tape were identified with new AF/flutter.
Discussion
The most recent ESC guidelines (2016) recommend short-term ECG recording followed by continuous monitoring for at least 72 hours in patients with TIA or ischaemic stroke.5 The aim of our study was to identify what percentage of patients with stroke or TIA get referred for prolonged rhythm monitoring in order to identify AF/flutter as a cause of their stroke. Despite our expectations, we did not identify any patients who had been referred for prolonged rhythm monitoring. We also identified that a significant proportion of patients did not even have a 24-hour tape; 24.5% of patients admitted with a stroke and 41% of patients diagnosed with TIA in the TIA clinic. The 24.5% of inpatients with a stroke might be an overestimate as we suspect that some of these patients would have had telemetry even if it was not mentioned in the discharge letter or documented in clinical notes. However, the 41% of outpatients diagnosed with TIA is an accurate and unexpectedly high percentage.
It is always a difficult task to translate the evidence from clinical trials into daily clinical practice, which is influenced by multiple variables and the desirable clinical judgement. We would propose to consider an implantable loop recorder in patients with ischaemic stroke or TIA who are less than 60 years old and have normal ECG, 24-hour tape and US/MRA of the carotids, especially if they have recurrent episodes or multiple territories are involved. If we apply these recommendations in our population we will identify 10 patients. Our audit period was three months, which would translate into 40 extra implantable loop recorders per year in our centre.
Another important finding of our study was that there was a clear tendency for patients diagnosed with lacunar strokes not to be referred for 24-hour tape. Analysis of the subgroup of patients with lacunar strokes who underwent rhythm monitoring, showed that similar percentages of patients were identified with AF/flutter when compared to the group of patients with non-lacunar strokes. Importantly though, we have demonstrated that the subgroup of patients with lacunar strokes had very similar patient characteristics and risk factors for stroke as the subgroup of patients with non-lacunar strokes. The pathogenesis of lacunar strokes and their relationship with AF/flutter is the subject of an ongoing debate in literature.12,13 As mentioned above, the main focus of stroke management should be the identification of modifiable risk factors even when the mechanism of a particular stroke is not certain. As the previous guidelines (ESC 2010, National Institute for Health and Care Excellence [NICE] 2014) and the most recent guidelines (ESC 2016) do not differentiate between ischaemic stroke subtypes when considering anticoagulation in patients with AF, then perhaps an equal effort should be made to identify AF in patients with an ischaemic stroke irrespective of the stroke subtype. Even if the exact cause of stroke might not be found with certainty, identifying and treating AF in stroke survivors will reduce the risk of subsequent strokes.
Key messages
- Real world data from our medium-sized district general hospital showed that no patients were referred for prolonged rhythm monitoring post-stroke
- A significant proportion of patients with a stroke do not get investigated for possible atrial fibrillation (AF)
- There is a tendency for patients with lacunar strokes not to get investigated for possible AF, even though the identification rate of AF is similar in patients with lacunar and non-lacunar strokes
Conflict of interest
None declared.
Funding
No funding to declare.
References
1. Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol 2014;6:213–20. https://doi.org/10.2147/CLEP.S47385
2. Lin HJ, Wolf PA, Kelly-Hayes M et al. Stroke severity in atrial fibrillation. Stroke 1996;27:1760–4. https://doi.org/10.1161/01.STR.27.10.1760
3. van Walraven C, Hart RG, Singer DE. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation. JAMA 2002;288:2441–8. https://doi.org/10.1001/jama.288.19.2441
4. Kernan WN, Ovbiagele B, Black HR et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160–236. https://doi.org/10.1161/STR.0000000000000024
5. Kirchhof P, Benussi S, Kotecha D et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. https://doi.org/10.1093/eurheartj/ehw210
6. Albers GW, Bernstein RA, Brachmann J et al. Heart rhythm monitoring strategies for cryptogenic stroke: 2015 diagnostics and monitoring stroke focus group report. J Am Heart Assoc 2016;5:e002944. https://doi.org/10.1161/JAHA.115.002944
7. Gladstone DJ, Spring M, Dorian P et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467–77. https://doi.org/10.1056/NEJMoa1311376
8. Gladstone DJ, Dorian P, Spring M et al. Atrial premature beats predict atrial fibrillation in cryptogenic stroke: results from the EMBRACE trial. Stroke 2015;46:936–41. https://doi.org/10.1161/STROKEAHA.115.008714
9. Sanna T, Diener H-C, Passman RS et al. Cryptogenic stroke and underlying atrial fibrillation: supplementary appendix. N Engl J Med 2014;370:2478–86. https://doi.org/10.1056/NEJMoa1313600
10. Thijs VN, Brachman J, Morillo CA et al. Predictors for atrial fibrillation detection after cryptogenic stroke: results from CRYSTAL AF. Neurology 2016;86:261–9. https://doi.org/10.1212/WNL.0000000000002282
11. Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass screening for untreated atrial fibrillation the STROKESTOP study. Circulation 2015;131:2176–84. https://doi.org/10.1161/CIRCULATIONAHA.114.014343
12. Wardlaw JM. What causes lacunar stroke? J Neurol Neurosurg Psychiatry 2005;76:617–19. https://doi.org/10.1136/jnnp.2004.039982
13. Park YS, Chung PW, Kim YB et al. Small deep infarction in patients with atrial fibrillation: evidence of lacunar pathogenesis. Cerebrovasc Dis 2013;36:205–10. https://doi.org/10.1159/000353736

