A 55-year-old man with suspected community-acquired pneumonia and atrial fibrillation was found to have a very large left atrial myxoma complicated with a pulmonary triad – pulmonary hypertension, pulmonary infarction, and pulmonary lymphadenopathy. The myxoma was successfully removed and complete resolution of all three pulmonary complications followed. He re-presented two weeks post-surgery with atrial flutter, which was medically treated and considered for ablation. We have taken the opportunity to undergo a mini-literature review on myxoma and its pulmonary complications.
Introduction
Myxoma is the most common primary cardiac tumour, found in the left atrium in 75% of cases.1,2 Clinical features range from being asymptomatic to symptoms of mitral stenosis, embolisation and systemic illness.3-5 Pulmonary complications, including pulmonary hypertension,4,6,7 pulmonary infarction8 and lymphadenopathy,9 though uncommon, have been reported. Our patient presented with a pulmonary triad of all the complications mentioned above and all of them resolved immediately following successful excision of the tumour.
We hope to report this case for educational and clinical purposes, due to the unusual combination of pulmonary complications, as well as atrial arrhythmias associated with a left atrial myxoma.
Case report
A 55-year-old man had presented to his general practitioner (GP) with a nine-month history of unexplained breathlessness on exertion and a four-week history of non-productive cough. He had been treated for an upper respiratory tract infection. Despite the treatment, his symptoms had worsened, causing him shortness of breath at rest and an irregular pulse, hence, his GP referred him urgently to the hospital.
His past medical history included multiple surgeries for bilateral ankle misalignment since childhood, the last of which had been performed three years prior and was complicated by a post-operative deep vein thrombosis (DVT). He had suffered with epilepsy from the age of 7 to 13 years.
On admission, he was not on any regular medication. His family history was silent for heart diseases or sudden death.
On examination, the patient was breathless at rest although his oxygen saturation was normal (98% on room air). He was tachycardic at 120 bpm with an irregularly irregular pulse. This was confirmed to be atrial fibrillation on a 12-lead electrocardiogram (ECG).
Chest examination revealed normal heart sounds with an early diastolic murmur, best heard over the mitral area, along with a late systolic murmur at the left sternal edge. His lung fields were clear, abdomen examination was unremarkable and there was no peripheral oedema.
His only blood test abnormality was a raised C-reactive protein (CRP) at 84 mg/L. His chest X-ray showed patchy opacities in the left and right middle and lower zones, along with Kerley B-lines, suggestive of pulmonary oedema.
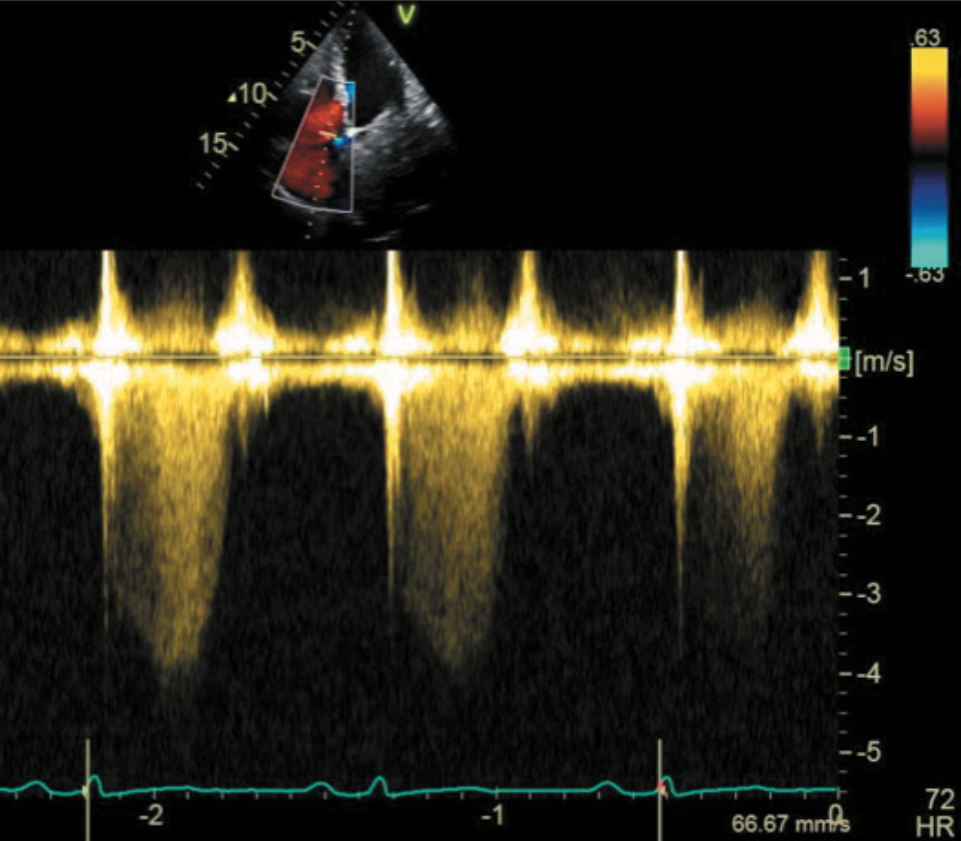
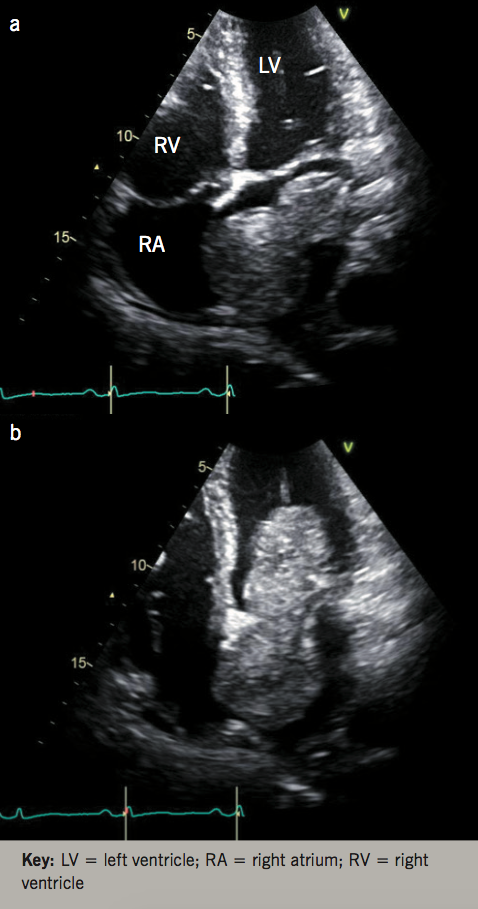
Transthoracic echocardiogram (TTE) revealed a left ventricle (LV) size at the upper limits of the normal range, with normal systolic function. The TTE also revealed a 96 x 44 mm highly mobile mass in the left atrium (LA) attaching to and involving the inter-atrial septum, with typical features of left atrial myxoma (figure 1). Nearly half of the mass was slipping into the LV through the mitral valve (MV) during the diastole, mimicking severe mitral stenosis (figures 1a and 1b). The systolic pressure in the pulmonary artery was markedly elevated to over 65 mmHg, estimated by continuous-wave Doppler of the tricuspid regurgitation (figure 2).
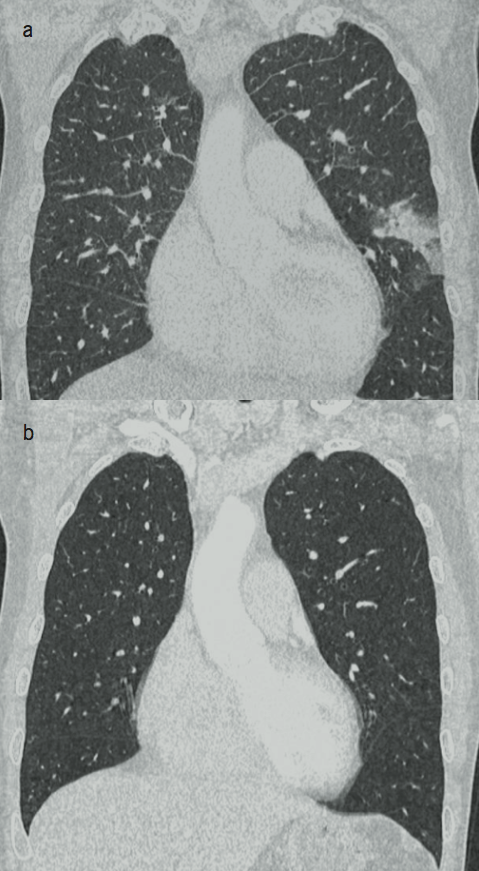
A chest computed tomography (CT) was performed and showed a filling defect in the LA, suggestive of a large intra-cardiac mass, hence, confirming the echocardiographic finding and the diagnosis of LA myxoma. The scan also demonstrated moderate bilateral pleural effusion, possible pulmonary oedema, mediastinal and bi-hilar lymphadenopathy, and a focal lingular peripheral opacification in the left lung field suggestive of a pulmonary infarct (figure 3a). The patient underwent a cardiac CT angiogram that showed no coronary disease.
After the diagnosis of a large LA myxoma was established, the patient was treated medically with metoprolol and digoxin for atrial fibrillation, and benzyl penicillin and clarithromycin for suspected community-acquired pneumonia.
The patient was promptly transferred to our local cardiothoracic centre for surgical removal of the myxoma. A 94 x 64 x 46 mm mass was completely excised along with a sizable portion of the atrial septum via the right atrium. The histology confirmed it as true atrial myxoma with no cellular atypia.
After surgery, his atrial fibrillation was converted to sinus rhythm, but he re-presented to us two weeks later with symptoms of breathlessness and reduced exercise tolerance, and was found to be in atrial flutter. A regimen of rate control medications and anticoagulation was started with a consideration for ablation. A repeat chest CT six weeks after the cardiac surgery confirmed the complete resolution of pulmonary oedema, mediastinal and hilar lymphadenopathy and pulmonary infarction (figure 3b). A repeat echocardiogram showed normalised LV and LA, and no more evidence of pulmonary hypertension.
Discussion
Primary cardiac tumours are rare, with an incidence reported to be below 0.03%.1 By comparison, metastatic involvement of the heart is significantly more frequent and has been reported in up to one in five patients who died of cancer on autopsy.1 Of all the primary cardiac tumours, myxoma is the most common (70–80%).9 Myxomas tend to be more frequently seen between the third and sixth decades of life, with a female-to-male ratio of 2:1 to 3:1.1,2,10
About 75% of all myxomas occur in the LA and usually originate from the interatrial septum in the fossa ovale region; less than a fifth are found in the right atrium, and 5–8% in the ventricles.5 Other locations are reported, though much rarer.1
The size of a myxoma can vary from a few millimetres to 15 cm, with an averaged maximal dimension of 3.7 to 5.6 cm.10 Therefore, the myxoma found in our patient was far larger than most reported and, thus, can be classified as giant or colossal.
The clinical features of a cardiac myxoma are related to its location, size and mobility.
The tumour can present with one or more symptoms of the three categories: outflow obstruction of the mitral valve, embolism and constitutional or systemic symptoms.4,5,12 In our patient, embolism manifested as pulmonary infarction.
The most common symptoms of LA myxomas are related to mitral valve obstruction (>60%): dizziness or syncope, palpitations, dyspnoea, cough, pulmonary oedema, or congestive heart failure. Embolic manifestations are less common (16%). A similar percentage of patients (15%) may present with systemic or constitutional symptoms such as myalgia, muscle weakness, arthralgia, fever, weight loss, fatigue, and even Raynaud syndrome.12
It would appear that the incidence of myxoma does not change with time on longitudinal observations, but the detection of it has become more frequent, likely due to the easy access and wide availability of echocardiography.10 The fact that our patient presented to us rather late, and the large myxoma size, suggested a long period of growth. Hence, he had developed a wide range of symptoms along with the pulmonary complications.
Atrial fibrillation has been described as a complication of a LA myxoma,9,10,12 and it can be a consequence of either the myxoma itself or the surgical procedure. In the former case, atrial fibrillation is resolved after a successful operation, but it remains difficult to ascertain a clear association between the two, although such association is well known between rheumatic mitral stenosis and atrial fibrillation. It would not be illogical to state that the large myxoma in our patient caused significant mitral valve obstruction (figure 1) and lead to the initial atrial fibrillation, and even subsequent atrial flutter after surgery.
The diagnosis of cardiac myxoma is usually made by imaging. TTE is generally the test of choice, with transoesophageal echocardiography (TOE) being used for more accurate assessment of morphology. Cardiac CT and magnetic resonance imaging (MRI) are increasingly used nowadays for the confirmation of the diagnosis and tissue characterisation of intracardiac masses. Nevertheless, the definitive diagnosis of intracardiac mass can only be achieved by histological examination following surgical excision, showing either myxoma or other tumours, thrombus, vegetation or cysts.5,13,14
As expected, once the diagnosis of left atrial myxoma was established, our patient had prompt surgical excision. Histology confirmed, not only the diagnosis of myxoma, but also the complete removal of the myxoma along with some myocardium. The surgical intervention is the only effective treatment and is considered relatively safe. Its outcome is excellent in all age groups, with an extremely low rate of re-occurrence in the long term.11,12,15
Finally, our case presented with all pulmonary complications of LA myxoma, a triad of pulmonary hypertension, pulmonary infarction and lymphadenopathy. These abnormalities resolved rapidly after surgical removal of the myxoma. The mechanism of their development and resolution is clearly based on the large myxoma itself. We would hypothesise that the pulmonary infarction was due to the invasion of the LA myxoma into the right atrium and myxoma fragments shed off to the pulmonary artery, similar to the reported case of a patient with right atrial myxoma;8 pulmonary hypertension was the result of pulmonary infarction or recurrent pulmonary embolism, while the pulmonary lymphadenopathy could be the reaction to the inflammation process, which was reported to be associated with high levels of interleukin (IL)-6 and erythrocyte sedimentation rate (ESR) in patients with myxoma.16
LA myxoma can present with one or two pulmonary complications. Our patient presented all three pulmonary complications, a pulmonary triad, which is probably uncommon. Prompt surgical excision resulted in complete resolution of all the pulmonary complications although the atrial arrhythmia remained to be treated.
Conflict of interest
None declared.
References
1. Basso C, Rizzo S, Valente M, Thiene G. Prevalence and pathology of primary cardiac tumors. Cardiovasc Med 2012;15:18–28. https://doi.org/10.4414/cvm.2012.01638
2. Yu K, Liu Y, Wang H, Hu S, Long C. Epidemiological and pathological characteristics of cardiac tumors: a clinical study of 242 cases. Interact Cardiovasc Thorac Surg 2007;6:636–9. https://doi.org/10.1510/icvts.2007.156554
3. Goodwin JF. Diagnosis of left atrial myxoma. Lancet 1963;281:464–8. https://doi.org/10.1016/S0140-6736(63)92359-6
4. Peters MN, Hall RJ, Cooley DA, Leachman RD, Garcia E. The clinical syndrome of atrial myxoma. JAMA 1974;230:695–701. https://doi.org/10.1001/jama.1974.03240050023018
5. Burke AP, Virmani R. Cardiac myxoma: a clinicopathologic study. Am J Clin Pathol 1993;100:671–80. https://doi.org/10.1093/ajcp/100.6.671
6. Akintunde AA. Giant left atrial myxoma presenting with heart failure and pulmonary hypertension in a middle-age Nigerian man. Curr Res Cardiol 2016;3:60–2. https://doi.org/10.4172/2368-0512.1000068
7. Brant LC, Mitu O, Gomide L, Bráulio R, Nunes MC. Large atrial myxoma causing mitral obstruction and severe pulmonary hypertension. J Heart Valve Dis 2011;20:357–9.
8. Aydın C, Taşal A, Ay Y, Vatankulu MA, İnan B, Bacaksız A. A giant right atrial villous myxoma with simultaneous pulmonary embolism. Int J Surg Case Rep 2014;5:206–08. https://doi.org/10.1016/j.ijscr.2013.11.014
9. Amano J, Kono T, Wada Y et al. Cardiac myxoma: its origin and tumor characteristics. Ann Thorac Cardiovasc Surg 2003;9:215–21.
10. Yuda S, Nakatani S, Yutani C, Yamagishi M, Kitamura S, Miyatake K. Trends in the clinical and morphological characteristics of cardiac myxoma. Circ J 2002;66:1008–13. https://doi.org/10.1253/circj.66.1008
11. Keeling IM, Oberwalder P, Anelli-Monti M et al. Cardiac myxomas: 24 years of experience in 49 patients. Eur J Cardiothorac Surg 2002;22:971–7. https://doi.org/10.1016/S1010-7940(02)00592-4
12. Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine 2001;80:159–72. https://doi.org/10.1097/00005792-200105000-00002
13. Scheffel H, Baumueller S, Stolzmann P et al. Atrial myxomas and thrombi: comparison of imaging features on CT. Am J Roentgenol 2009;192:639–45. https://doi.org/10.2214/AJR.08.1694
14. Abbas A, Garfath-Cox KAG, Brown IW, Shambrook JS, Peebles CR, Harden SP. Cardiac MR assessment of cardiac myxomas. Br J Radiol 2015;88:20140599. https://doi.org/10.1259/bjr.20140599
15. Bossert T, Gummert JF, Battellini R et al. Surgical experience with 77 primary cardiac tumors. Interact Cardiovasc Thorac Surg 2005;4:311–15. https://doi.org/10.1510/icvts.2004.103044
16. Kuroki S, Naitoh K, Katoh O, Yamada H, Itoh T. Increased interleukin-6 activity in cardiac myxoma with mediastinal lymphadenopathy. Intern Med 1992;31:1207–09. https://doi.org/10.2169/internalmedicine.31.1207
