A. Menarini Farmaceutica Internazionale SRL has provided an educational grant for the production of this e-learning programme and has had no editorial control or input. The views and content expressed within this programme are solely those of the authors.
PP-CA-UK-0153 Date of preparation: March 2020
Presentation
Chest pain is very common with 20-40% of the general population experiencing it during their lives. In the UK, chest pain accounts for up to 1% of visits to general practitioners (GPs), approximately 700,000 visits (5%) to emergency departments and up to 40% of emergency admissions to hospital.1,2 Importantly, in patients with chest pain due to acute coronary syndromes (ACS) or angina, effective treatments exist to improve morbidity and mortality. This module will focus on chronic coronary syndrome and will not review the clinical syndromes associated with ACS.
Table 1. Clinical assessment
| Clinical history | |
|---|---|
| Record: | Age and sex |
| Pain characteristics, factors provoking and relieving the pain | |
| Associated symptoms | |
| History of CVD | |
| Cardiovascular risk | |
| Physical examination | |
| Identify cardiovascular risk factors | |
| Look for signs of other CVD | |
| Exclude: | Non-coronary causes of angina (e.g. severe aortic stenosis, cardiomyopathy) |
| Other causes of chest pain | |
| Key: CVD = cardiovascular disease Adapted from NICE6 |
|
Rapid access chest pain clinics (RACPC) have been developed to quickly assess patients with new onset angina from suspected coronary artery disease (CAD).
Around 50% of patients admitted with chest pain to hospital have a non-cardiac cause of their symptoms. Many can be better evaluated in a RACPC rather than an emergency department.3
Whatever the setting, the following protocol (see table 1) should be adopted when investigating chest pain, as research has shown that chest pain may have different aetiologies depending on where patients present. The cornerstone of a diagnosis of angina is a careful history and subsequent physical examination and investigations are used to confirm the diagnosis, exclude alternative causes and risk stratify. The clinical assessment of angina is discussed in detail in the subsequent sections.
Causes of chest pain
Since there are many causes of chest pain, it is important to differentiate the various causes and confirm or exclude cardiovascular causes. Some of the cardiovascular and non-cardiovascular causes of chest pain are shown in table 2.
Table 2. Cardiovascular and non-cardiovascular causes of chest pain
| Cardiovascular | |
|---|---|
| Ischaemic | Flow-limiting coronary stenosis |
| Coronary vasospasm | |
| Coronary thrombosis | |
| Non-ischaemic | Coronary arterial wall distension |
| Dyssynergic myocardial contraction | |
| Aortic dissection | |
| Pericarditis | |
| Pulmonary embolism | |
| Hypertension | |
| Non-cardiovascular | |
| Gastrointestinal | Oesophageal spasm |
| Gastro-oesophageal reflux | |
| Gastritis/duodenitis | |
| Peptic ulcer | |
| Cholecystitis | |
| Respiratory | Pleuritis |
| Mediastinitis | |
| Pneumothorax | |
| Neuromuscular/ skeletal |
Chest wall pain syndrome |
| Neuritis/radiculitis | |
| Herpes zoster | |
| Tietze’s syndrome | |
| Psychogenic | Anxiety |
| Depression | |
The pre-test probability of the underlying cause of chest pain varies depending on whether a patient:
- is seen by a GP,
- calls the dispatch centre,
- is treated by the ambulance crew or
- is seen in the emergency department.
The distribution of aetiologies in relation to these four scenarios is shown in table 3. Not unexpectedly, chest pain of cardiac origin is less commonly seen by the GP (20%), whereas musculoskeletal disorders are common.3
Table 3. Aetiology to chest pain in various clinical settings
| Aetiology | General practitioner (1–3)% | Dispatch centre (4)% | Ambulance crew (5)% | Emergency department (6)% |
|---|---|---|---|---|
| Cardiac | 20 | 60 | 69 | 45 |
| Musculoskeletal | 43 | 6 | 5 | 14 |
| Pulmonary | 4 | 4 | 4 | 5 |
| Gastrointestinal | 5 | 6 | 3 | 6 |
| Psychiatric | 11 | 5 | 5 | 8 |
| Other | 16 | 19 | 18 | 26 |
| Reproduced with kind permission from Erhardt et al.3 | ||||
As seen above, the most common causes of chest pain seen in the GP surgery are non-cardiac (table 2).4 They are usually differentiated by careful history taking.
Anginal pain
Typical angina meets all three characteristics5 of:
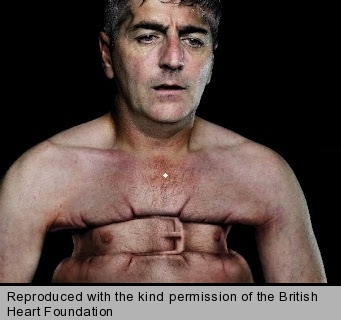
- constricting discomfort in the front of the chest (figure 1), neck, jaw, shoulder or arm
- being precipitated by physical exertion
- relieved by rest or glyceryl trinitrate (GTN) within five minutes.
Table 4. Clinical classification of chest pain
| Typical angina (definite) | Meets three of the following characteristics: |
|---|---|
| Substernal chest discomfort of characteristic quality and duration | |
| Provoked by exertion or emotional stress | |
| Relieved by rest and/or glyceryl trinitrate spray | |
| – | |
| Atypical angina (possible) | Meets two of these characteristics |
| – | |
| Non-cardiac chest pain | Meets one or none of these characteristics |
| Adapted from Allamsetty et al.4 | |
Atypical angina meets two of these characteristics whilst non-anginal chest pain meets only one, or none, of these (table 4).
The National Institute for Health and Care Excellence (NICE) advises that the definitions of typical, atypical and non-anginal chest pain do not differ between men and women, nor between ethnic groups.

Factors that make stable angina more likely include:
- increasing age
- male gender (figure 2)
- presence of cardiovascular risk factors
- other cardiovascular disease
- history of established coronary artery disease (CAD) e.g. previous myocardial infarction (MI), coronary revascularisation.6
Factors that make stable angina less likely include:6
- continuous or very prolonged
- unrelated to activity
- brought on by inspiration
- associated with dizziness, palpitations, tingling or difficulty swallowing.
Be aware that in certain groups the pain may present differently. A 2014 consensus statement from the American Heart Association7 highlights that women have a broader spectrum of presenting symptoms. They have a different pattern and distribution of non-chest-related pain symptoms. Compared to men, women’s ischaemic symptoms are more often precipitated by mental or emotional stress and less frequently by physical exertion. Women also more often report epigastric discomfort and associated nausea, radiation of discomfort to the arms, neck and interscapular areas, and dyspnoea and fatigue.
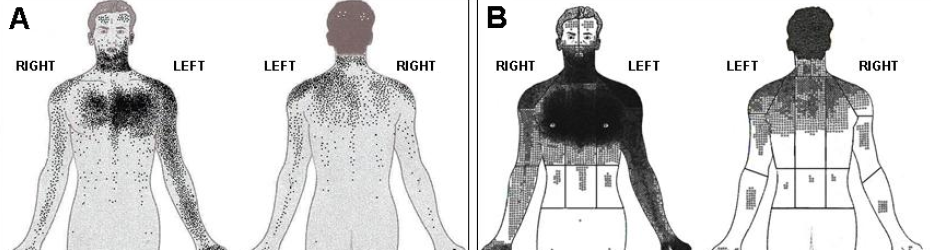
A study conducted in London has highlighted that Asian patients tend to be younger, more likely to have diabetes and tend to report discomfort over a greater area of their body with a greater incidence of posterior chest discomfort than white Europeans (see figure 3).8 South Asian women tend to report a wider distribution of discomfort and intensity than men across all subgroups.8
The Canadian Cardiovascular Society has provided a grading system which is commonly used to stratify the severity of angina (table 5).9
Table 5. Canadian Cardiovascular Society (CCS) grading of angina pectoris
| Grade | Description | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Ordinary physical activity does not cause angina, such as walking and climbing stairs. Angina with strenuous or rapid prolonged exertion at work or recreation. | ||||||||
| Grade 2 | Slight limitation of ordinary activity. Walking or climbing stairs rapidly, walking uphill, walking or stair climbing after meals, or in cold, in wind or under emotional stress, or only during the few hours after awakening. Walking more than two blocks on the level and climbing more than one flight of ordinary stairs at a normal pace and in normal conditions. | ||||||||
| Grade 3 | Marked limitation of ordinary physical activity. Walking one or two blocks on the level and climbing one flight of stairs in normal conditions and at a normal pace. | ||||||||
| Grade 4 | Inability to carry out any physical activity without discomfort; angina may be present at rest. There are four sub-groups in CCS grade 4. Groups A to D:
|
||||||||
| Data from Canadian Cardiovascular Society9 | |||||||||
Common differentials for anginal pain
Differentials for angina in the patient presenting with chest pain include the following conditions (table 6).
Table 6. Common differentials for anginal pain
| Differential | Physical exam findings | Definitive test |
|---|---|---|
| Acute coronary syndromes | Shortness of breath, weakness, nausea, vomiting, palpitations, syncope | ECG, biochemical markers |
| Aortic aneurysm | Pain pleuritic in origin, different blood pressure readings in upper limbs | CT, TOE |
| Cholecystitis | Tender epigastrium/RUQ | Abdominal ultrasound |
| Gastroesophageal disease | Epigastric tenderness | Endoscopy |
| Herpes zoster | Typical rash which ranges from maculopapular to vesicular | This is a clinical diagnosis |
| Musculoskeletal pain | Focally tender area on chest | Physical exam, CK readings but this is a clinical diagnosis |
| Myocarditis | Frequently normal, signs of CCF, S3 | Echo and cardiac MRI |
| Oesophageal rupture | Pain retrosternal in origin, significant vomiting, general unwell | X-ray |
| Pancreatitis | Pain abdominal (epigastric) in origin, tenderness | Serum amylase |
| Pericarditis | Dull heart sounds, evidence of viral infection | ECG and echocardiography but this is a clinical diagnosis |
| Pneumonia | Crepitations, decreased air entry and wheeze in affected zones | Chest X-ray, CT thorax |
| Pulmonary embolism | Sinus tachycardia, signs of right ventricular strain | CT pulmonary angiogram |
| Spontaneous pneumothorax | Decreased air entry, tympanic percussion note | Chest X-ray, CT thorax |
| Valvular heart disease | Characteristic murmur and altered haemodynamics | Echocardiography |
| Key: CT = computed tomography; CCF = congestive cardiac failure; CK = creative kinase; ECG = electrocardiogram; MRI = magnetic resonance imaging; RUQ = right upper quadrant; S3 = third heart sound; TOE = transoesophageal echocardiography | ||
Acute coronary syndromes (ACS)
Anginal pain that is new-onset, at rest or progressive should raise the suspicion of ACS. Atypical features should also be considered in women, patients with diabetes and younger patients, including symptoms such as shortness of breath, weakness, nausea and vomiting, palpitations and syncope. An urgent electrocardiogram (ECG) should be performed and the patient transferred to the emergency department or local Heart Attack Centre as appropriate.10
Pulmonary embolism (PE)
Common symptoms include shortness of breath, pleuritic chest pain, cough and symptoms of deep venous thrombosis (DVT). On examination, patients may have a resting tachycardia, evidence of DVT, risk factors for thrombosis and may be hypoxic. Auscultation of the chest is often normal. Risk scores such as Well’s score should be used to assess pre-test probability and an ECG should be performed to detect sinus tachycardia, S1Q3T3 pattern, or signs of right ventricular (RV) strain.
Pericarditis
Commonly after a viral infection, this is characterised by a triad of:
- pleuritic chest pain that may improve on sitting forward
- friction rub on auscultation
- characteristic ECG changes.
ECG changes include upwardly concave ST elevation, which is usually widespread with no reciprocal changes apart from in AVR and V1. The PR segments may also be depressed. Low voltage ECG may be present in patients with an associated pericardial effusion. Together with myocarditis, a wide differential should be considered, such as infection, drug-related, autoimmune, uraemia and malignancy. A concurrent (or recent) fever may be present.
Valvular heart disease
Valvular dysfunction can lead to anginal-type pain due to a compromised cardiac output as well as related pulmonary hypertension and arrhythmia. Causes include aortic stenosis, mitral stenosis and aortic incompetence. The most obvious clinical finding will be altered haemodynamics and characteristic murmurs (see below for details).
Myocarditis
Patients with myocarditis may be asymptomatic or have symptoms of fatigue, palpitations, chest discomfort or heart failure. A tachycardia or S3 gallop may be noted. The ECG may show ST changes, arrhythmias or heart block. An echocardiogram and/or magnetic resonance imaging are diagnostic in the context of elevated troponin levels on blood tests.
Aortic aneurysm
This is a severe sharp pain located in the chest (ascending aorta) and frequently in the back (aortic arch and descending aorta). Pleuritic in origin and associated with a ripping or tearing sensation if recently dissected, it may cause differential blood pressure (BP) readings in the upper limbs. It requires a strong index of suspicion and requires computed tomography (CT) or transoesophageal echocardiography (TOE) for diagnosis. Myocardial ischaemia in a coronary territory may be present if the dissection extends to the coronary ostia and affects coronary blood flow.
Gastro-oesophageal disease/oesophagitis
Typically, this has a burning quality, with radiation to the base of the neck or into the back that may be associated with a waterbrash sensation (regurgitation of excessive saliva associated with some acid). Epigastric tenderness may be present clinically and symptoms may worsen on lying supine, especially if a hiatus hernia is associated. It may be difficult to definitively differentiate between gastro-oesophageal disease and angina. Response to antacids or acid-reducing medications (e.g. proton-pump inhibitors or H2-receptor antagonists such as ranitidine) may be suggestive of aetiology. An oesophago-gastro-duodenoscopy should be considered, especially if dyspepsia exists in the presence of other red flag symptoms.11
Oesophageal rupture/perforation
In the setting of significant vomiting and severe retrosternal chest pain, the diagnosis of oesophageal rupture/perforation (also known as Boerhaave’s syndrome) should be considered. Patients may be systemically unwell and a chest X-ray may show a pneumomediastinum. Urgent referral should be considered for this life-threatening condition.
Spontaneous pneumothorax
Sudden onset pleuritic pain and shortness of breath associated with absent breath sounds, tympanic percussion note and hypoxia suggests this diagnosis. Haemodynamic compromise (such as hypotension, tachycardia) are ominous features that suggest a tension pneumothorax which may require emergent treatment. In cases of simple pneumothorax without haemodynamic compromise, an urgent plain inspiratory posterior anterior chest X-ray will reveal the diagnosis.
Musculoskeletal pain
This typically has an associated history that suggests the cause, e.g. lifting a heavy object, undertaking exercise beyond usual habits, trauma or arthritic symptoms. The history may not be readily apparent in these patients and a detailed exercise, sport and occupational history may be helpful to elucidate underlying provoking factors. Palpation of the chest for provocation of costochondritic pain should be undertaken routinely in all chest pain patients who do not have a clear diagnosis. Costochondritis is a common cause. Other important differentials should be excluded before a diagnosis of musculoskeletal chest pain is reached.
Cholecystitis
The diagnosis is suggested by right upper quadrant tenderness, positive Murphy’s sign and can be confirmed on ultrasound.
Pancreatitis
Central/epigastric abdominal pain and tenderness, which is worse when lying supine. Commonly associated with a history of gallstones or alcohol excess. Serum amylase should be tested for.
Pneumonia
The presence of fever and cough (productive or non-productive) will easily discriminate between angina and the typically pleuritic pain of pneumonia. Patients will frequently describe bronchospasm as ‘a tightness’ and detailed questioning will help to differentiate this from angina.
Herpes zoster
Typical dermatomal distribution of maculopapular rashes or vesicular lesions will suggest the diagnosis here.
With all these differentials, the characterisation of the pain is useful. Pleural pain may be caused by infection, pulmonary embolism or tumour. The character of the pain is important with elucidation of pulmonary symptoms. Musculoskeletal pain, such as with Tietze’s syndrome (a benign inflammation of the costal cartilage), is suggested by an apleuritic character and local tenderness. Referred pain from the thoracic spine can be suggested by previous history, trauma and local tenderness. Biliary pain with epigastric or right hypochondrial discomfort, is worse with fatty foods and associated with nausea.4
Investigations in primary care
Low-risk patients with atypical symptoms may be managed in primary care where possible. Symptoms and concerns should be explained, and reassurance provided, where necessary. Correct management by GPs may both reduce morbidity and the need for referral.4
Where there is a low index of suspicion of coronary heart disease (CHD), then it is not recommended that the patient undergoes further tests.
GPs should ask the following questions (adapted from Schroeder12):
- The site and nature of the pain – sharp, dull, central, peripheral, any radiation?
- Its duration – was there a trigger, for example an injury, which triggered the pain? Severe chest pain for a couple of hours is often more significant than pain that has been present for weeks.
- Are there any associated symptoms – such as breathlessness, fever, cough.
- Are there provoking and relieving factors – is the pain worse on inspiration? Is it worse on movement? Is it brought on by exertion and relieved by rest? Any response to nitrates?
- Past medical history – DVT, immobility or long-distance travel, recent surgery, clotting tendencies, respiratory disease such as asthma or chronic obstructive pulmonary disease, cardiovascular disease, hyperlipidaemia, hypertension, diabetes.
- Family history – cardiovascular disease, clotting tendencies.
- Drug history – cholesterol-lowering drugs, thrombogenic medication such as the combined oral contraceptive, consider asking about recreational drugs.
- Smoking history.
Cardiovascular risk scores may help GPs guide management and prevention. Scores include QRISK2 and JBS3. NICE guidelines have stratified management of patients with a cardiovascular disease risk score of i) less than 10% or ii) 10% or more using QRISK2.13
Assessment of chest pain in primary care
General physical examination
Physical examination is useful for excluding important differentials since examination is frequently normal in stable angina. Important differentials to exclude include:
Life-threatening conditions:
- Pulmonary embolism
- Examination is often remarkable. May have decreased air entry, pleural rub or crackles due to infarcted segment. Clinical signs of DVT may be present.
- Aortic dissection
- Significant difference in inter-arm BP. Diastolic murmur may be present from aortic incompetence.
- Cardiac tamponade
- Three signs are typical (Beck’s triad):
- hypotension
- elevated jugular venous pressure
- muffled heart sounds
- Pulsus paradoxus may be present – abnormally large decrease in systolic BP (>10mmHg) on inspiration)
- Pericardial rub if tamponade in the context of pericarditis.
- Three signs are typical (Beck’s triad):
- Oesophageal rupture
- A fever, tachycardia, tachypnoea and hypotension may develop. Subcutaneous or mediastinal emphysema (heard on auscultation).
- Tension pneumothorax
- Reduced chest expansion and breath sounds. Mediastinal shift and potential haemodynamic compromise.
Others:
- Dyspepsia/oesophagitis: epigastric tenderness on palpation.
- Biliary colic: positive Murphy’s sign and right upper quadrant tenderness.
- Pneumonia: coarse inspiratory crackles and bronchial breathing. A fever may be present as well as a productive cough.
- Simple pneumothorax: decreased or absent breath sounds and reduced chest expansion. Tympanic percussion note.
- Musculoskeletal pain: may be possible to provoke the index discomfort by applying pressure to the area of maximum discomfort or by carrying out manoeuvres to either utilise the involved muscle groups or apply a stress through the involved intercostal, neck or shoulder joints.
Table 7. What to include in patient examination
| Weight, height and calculation of body mass index (BMI) |
| Waist circumference to evaluate presence of the metabolic syndrome |
| Pulse rate and rhythm |
| Blood pressure |
| Presence of murmurs, especially aortic stenosis |
| Evidence of hyperlipidaemia with xanthelasma or tendon xanthomata |
| Evidence of peripheral vascular disease with absent foot pulses, bruits, skin changes, hair loss |
| Evidence of anaemia or thyroid disease |
| Data from Allamsetty4 |
The physical examination is an important step before referral (table 7) because it may identify conditions that can precipitate angina (such as anaemia or hyperthyroidism) and conditions other than that can present with angina (such as aortic stenosis/hypertrophic obstructive cardiomyopathy). There may also be findings that would make a treadmill test unsuitable, such as uncontrolled blood pressure or aortic stenosis. An example physical examination can be watched courtesy of NurseLedClinics.com
Cardiac examination
This is useful for detecting complications from advanced ischaemic heart disease and differential diagnoses for chest pain (e.g. aortic stenosis, hypotrophic cardiomyopathy) – caution as these are often subtle and easily missed!
Additional heart sounds
3rd heart sound
4th heart sound
Murmurs
The following constitute the most relevant murmurs in acute ischaemia:
- Mitral regurgitation. New mitral regurgitation is a very significant clinical finding as it can herald impending cardiac failure in ACS. Mechanisms include ischaemic papillary dysfunction, papillary rupture and regional wall hypokinesia/akinesia with secondary failure of valve leaflets to coapt sufficiently.
- Murmur of ventricular septal defect. This is described as a pansystolic murmur which is best appreciated at the left sternal border
- Occasionally a new aortic incompetence murmur will be appreciated in an aortic dissection which may also dissect along the coronaries producing a ST-elevation myocardial infarction (STEMI) and possibly complete heart block.
Example murmurs:
Mitral regurgitation
Aortic stenosis
Aortic incompetence
Ventricular septal defect
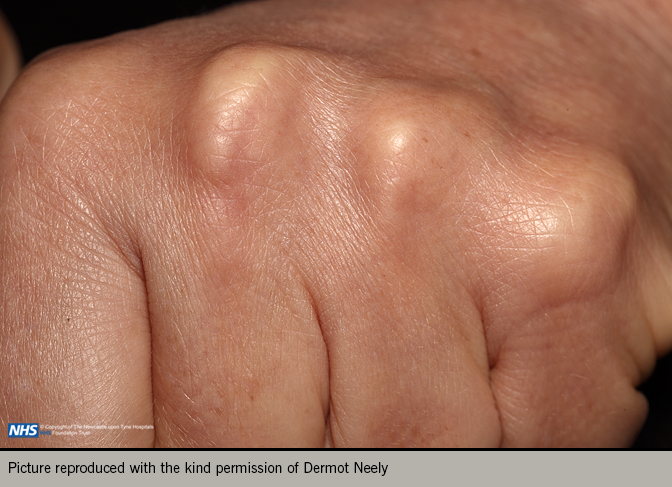
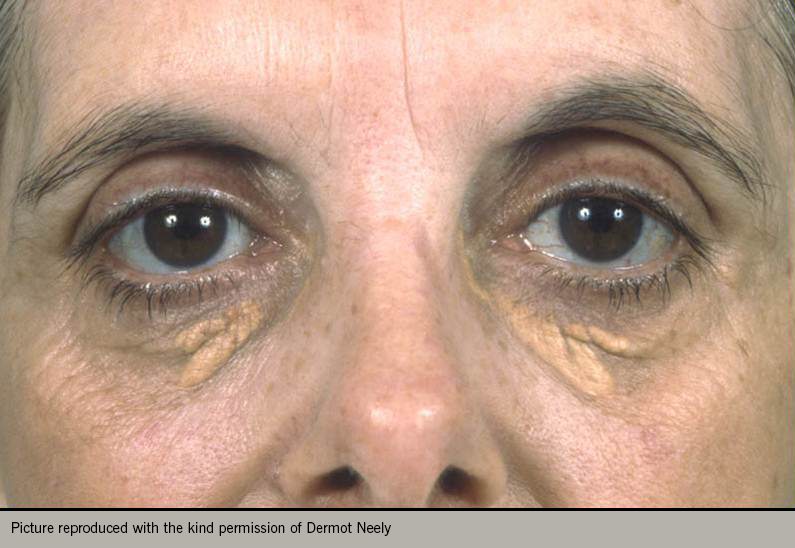
A comprehensive examination should include weight and height to allow calculation of body mass index, and waist circumference to evaluate presence of metabolic syndrome. Record the pulse rate, rhythm and blood pressure. Look for evidence of hyperlipidaemia with tendon xanthomata or xanthelasma (figures 4 and 5). Examine for evidence of peripheral vascular disease (palpation of peripheral pulses, auscultation of carotid and femoral arteries, and ankle-brachial index), bruits, skin changes or hair loss. Look for evidence of anaemia, thyroid disease renal disease or diabetes.5
Investigations:
Blood tests
The following patient blood tests are recommended by the 2019 European Society of Cardiology Guidelines (ESC) on Chronic Coronary Syndrome:5
- full blood count, including haemoglobin
- creatinine and estimated glomerular filtration rate
- lipid profile (fasting sample recommended), including total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides
- HbA1c and fasting plasma glucose measurements to screen for type 2 diabetes. Oral glucose tolerance test if above results inconclusive
- thyroid function tests if thyroid disorders suspected
- uric acid level should be considered
- high-sensitivity troponin T or I if suspicion of myocardial injury.
12-lead ECG
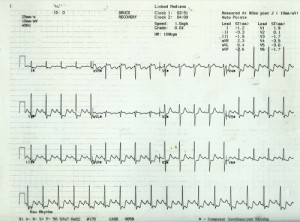
A resting 12-lead ECG (figure 6) should be recorded in anyone with suspected angina in order to provide information on heart rate, rhythm, the presence of previous myocardial damage (Q waves) and conduction defects (e.g. left bundle branch block or atrioventricular conduction block) that may be due to previous ischaemia. Atrial fibrillation is also a common finding in patients presenting with chest pain. It should be noted that ST depression in the context of supraventricular tachycardias are not predictive of CAD.
A normal resting ECG is not uncommon even in patients with severe angina but does not exclude the diagnosis of ischaemia. More than 50% of people with stable angina have a normal resting ECG.
As per the latest ESC Guidelines, a resting 12-lead ECG is recommended in all patients during or immediately after an episode of angina suspected to be due to CAD. If dynamic ischaemic changes are recorded during an episode of angina, these are crucial for the diagnosis. Ischaemic changes to be aware of in particular are:
- pathological Q waves
- left bundle branch block
- ST-segment and T wave abnormalities.
Some ECG abnormalities may exclude patients from a diagnostic treadmill test such as left bundle branch block and atrial fibrillation.
Long-term ambulatory ECG is rarely useful but may be considered in select patients with anginal episodes unrelated to exercise or those where silent myocardial ischaemia is suspected.
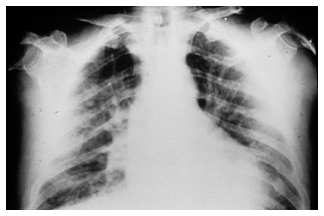
Chest radiograph (X-ray)
A chest radiograph is frequently used in the initial evaluation of chest pain, but for chronic coronary syndromes, it does not provide specific information for diagnosis or risk stratification.5 It is useful to assess other pathology, such as in the case of heart failure, pulmonary disease or valvular pathology (where features such as cardiomegaly, pulmonary congestion, or cardiac calcification may indicate a worse prognosis), or to exclude other causes in atypical presentations.
Echocardiography
An echocardiogram is a simple, cheap non-invasive test that provides useful information on cardiac function and anatomy. Reduced left ventricular function or the presence of regional wall motion abnormalities may increase the suspicion of ischaemic myocardial damage and the presence of CAD but left ventricular ejection fraction is often normal in patients with chronic coronary syndromes.14
Importantly, an echocardiogram is able to differentiate other causes of chest pain, e.g. valvular disease, and concurrent cardiac disease e.g. cardiac failure and cardiomyopathies.
Cardiac magnetic resonance (CMR)
CMR may be performed when an echocardiogram is inconclusive. It provides detailed information on cardiac structure, regional and global function, and with the use of gadolinium contrast can reveal fibrosis and evidence of previous myocardial injury. Stress perfusion CMR may be useful in the assessment of chronic coronary syndromes but its recommendation is not formalised in the ESC Guidelines.
NICE guidelines recommend the use of stress CMR or stress echocardiography for diagnosing stress-induced wall motion abnormalities. Furthermore, when considering which non-invasive test to offer (myocardial perfusion scintigraphy, stress echocardiography or CMR), NICE recommends taking account of locally available technology, expertise, the patient’s preferences and any contra-indications.6
CT coronary angiography
CT coronary angiography (CTCA) is a highly sensitive and specific tool for investigating the patient with stable chest pain to rule out significant underlying CAD. The patient-based diagnostic accuracy of quantitative CT angiography for detecting or ruling out stenoses of 50% or more according to conventional angiography reveals a sensitivity of 85% and a specificity of 90%.15
The pivotal SCOT-HEART trial (n=4, 146) investigating the use of CTCA in patients with suspected angina due to CAD demonstrated its ability to clarify the diagnosis and allow the targeting of appropriate interventions.16 It reclassified the diagnosis of CHD in 27% of patients, and the diagnosis of angina due to CHD in 23%. At five-year follow-up, patients who had CTCA had a significantly lower rate of death from CHD or nonfatal MI.
The 2016 update to the NICE guideline for the investigation of chest pain of recent onset has brought CTCA to the forefront as an initial investigation.6 This has been driven by favourable cost-utility analyses for CTCA. In an analysis of 16 different diagnostic strategies (including CTCA, cardiac MRI, echocardiography and nuclear tests), CTCA had the lowest cost per correct diagnosis due to its low cost, high sensitivity and negative predictive value, and low probability of complications.17
NICE recommend that in patients in whom stable angina cannot be excluded by clinical assessment alone, 64-slice (or above) CTCA should be offered if:
- clinical assessment indicates typical or atypical angina
- clinical assessment indicates non-anginal chest pain but 12-lead resting ECG indicates ST-T changes or Q waves.
Indeed, new ESC Guidelines support the initial use of CTCA and recommend that either CTCA or non-invasive functional imaging for ischaemia should be used as the initial test for diagnosing CAD in symptomatic patients.5 Furthermore, it is recommended that CTCA should be considered as an alternative to invasive angiography if another non-invasive test is equivocal or non-diagnostic.
CTCA offers a detailed non-invasive assessment of the coronary arteries and its use as an initial screening test is strongly supported by the NICE and ESC Guidelines. It is able to provide an angiographic view of the coronary arteries and, particularly through 3D reconstruction, is able to display complex coronary anatomy.
A feature available with CTCA is a calcium score that reflects that amount of calcified atherosclerotic lesions. Whilst the presence of calcium may indicate obstructive CAD, the absence of coronary calcium (known as an Agatston score of 0) is useful as it is associated with a low prevalence of obstructive CAD (<5%) and a low risk of death and non-fatal MI (<1% annual risk).14,18 It should be noted, however, that non-calcified obstructive coronary lesions may not be detected on CTCA and coronary calcium is only a weak predictor of obstructive CAD.
Consequently, a calcium score on CTCA should only be considered as a risk factor in the cardiovascular assessment of asymptomatic patients. It is not recommended to identify patients with obstructive CAD.
Furthermore, it is recommended that CTCA is not used in cases of:
- extensive coronary calcification
- irregular heart rate
- significant obesity
- inability to cooperate with breath-hold commands
- other conditions that may degrade image quality.
Although CTCA is sensitive for detecting obstructive CAD, lesions that are 50-90% by visual inspection may not necessarily be functionally significant so as to cause ischaemia. Consequently, non-invasive or invasive functional testing is recommended. Recently, advanced computational analysis has allowed the HeartFlow® system to predict the invasive fractional flow reserve (FFR) from standard CTCA acquisition enabling the cardiologist to gain a non-invasive functional, as well as anatomical assessment of CAD. It has shown promise in achieving similar clinical outcomes to usual testing and may be a cost-saving technology. For further discussion on HeartFlow® see Brady et al.19
Patients also need to be adequately counselled regarding the radiation exposure from CT scanning which is estimated (depending on the scanner) as being between 3 and 20 mSv (mean of 7).20 This radiation exposure dose has been falling with more modern scanners and with expert personnel. In the UK, the average patient dose for CTCA is 5.9 mSv.21
The excess lifetime cancer risk should be explained to patients although this is difficult to precisely quantify. For example, one study estimated that one in 270 women who undergo a CT coronary angiogram at age 40 years will eventually develop cancer, as compared with one in 600 men. The excess risks are approximately doubled in patients aged 20, but were approximately 50% lower for 60-year-olds.22 Another study has shown a much lower life attributable risk (LAR) or cancer incidence after CTCA.23 At a median age (62 years) of their case series (n=561) for radiation exposure from 1.33–3.81 mSv, LAR was 1 in 4,329 in men and 1 in 4,629 in women. For higher exposures 10.34–18.97 mSv at the same age, LAR was 1 in 1,336 in men and 1 in 614 in women. It should be noted, however, that the effective radiation dose of CTCA is similar to that of invasive angiography (2-23 mSv; mean of 7).20
Non-invasive functional imaging
If CTCA is non-diagnostic, functional imaging is recommended. The choice of non-invasive test depends on a number of factors, e.g. likelihood of CAD, patient characteristics affecting test performance, local expertise and availability of tests. Several options for functional imaging exist:
- myocardial perfusion scintigraphy with single photon emission computed tomography (MPS with SPECT)
- stress echocardiography
- first-pass contrast-enhanced magnetic resonance perfusion
- MR imaging for stress-induced wall motion abnormalities.
Exercise testing and limitations
Although exercise ECG is widely available and relatively cheap, it is no longer recommended by NICE as an initial test for suspected obstructive CAD (though it is still recommended in US and ESC guidelines). Exercise testing has been shown to have 78% sensitivity and 70% specificity for detecting CHD but compared with diagnostic imaging tests (e.g. CT coronary angiogram or functional imaging) has inferior diagnostic performance.24-6 The incremental prognostic power of exercise ECG over clinical assessment has also been shown to be poor.27
In addition, exercise ECG may be difficult to interpret in cases where an arrhythmia is present or in patients who may exercise sub-maximally. NICE Guidelines do not recommend that exercise ECG is used to diagnose or exclude stable angina for people without known CAD. For people with confirmed CAD (e.g. previous MI, revascularisation, previous angiography), NICE recommends non-invasive functional testing when there is uncertainty about whether chest pain is caused by myocardial ischaemia. An exercise ECG may be used instead of functional imaging.6
Specific scenarios however exist where an exercise ECG may be considered:
- As an alternative to rule-in or rule-out obstructive CAD when other non-invasive or invasive tests are not available
- To assess exercise tolerance, symptoms, arrhythmias, BP response and event risk in selected patients.
If there is uncertainty regarding symptomatology, exercise on the treadmill can be helpful,28 and the potentially therapeutic effect of a treadmill test followed by reassurance from an experienced healthcare professional should not be forgotten. If a test is to be used for these purposes, it is better that it is relatively cheap and without exposure to ionising radiation.
Holter monitoring
Developed from the pioneering work of American physicist Norman J Holter during the 1940s, ambulatory electrocardiography (figure 7) has come to play an important clinical and research role in the detection of cardiac arrhythmias and transient myocardial ischaemia (silent ischaemia). Ambulatory monitoring also plays an important role in the detection of cardiac arrhythmias and ventricular ectopic activity. This is an independent predictor of mortality for sudden and non-sudden death in patients with specific cardiac disorders. These include:
- ischaemic heart disease (particularly in the immediate post-MI period)
- hypertrophic and dilated cardiomyopathy
- post-operative congenital heart disease
- prolonged QT interval syndromes.
Over recent years, frequency modulated devices have become available and the use of Holter monitoring to detect myocardial ischaemia has increased.29 During the 1970s and 1980s, there was an explosion of interest in using ambulatory monitoring to study the patterns of ST segment changes in patients with stable and unstable coronary syndromes and Prinzmetal’s angina. It soon became apparent that the majority of episodes of ST segment depression were asymptomatic and had similar characteristics in terms of heart rate rise and duration to those episodes accompanied by angina. Treatment should therefore be aimed at the elimination of painful ischaemia as this will have the same effect on silent ischaemia.
Holter monitoring for ischaemia is currently most commonly used as an investigational tool in pharmaceutical trials, to evaluate the efficacy of new products, usually in comparison to placebo.29 It may reveal evidence of silent ischaemia but often does provide additional diagnostic or prognostic information that cannot be obtained from stress testing. Furthermore, ischaemic changes on ambulatory ECG monitoring are frequent in women but do not correlate with stress testing.30 It is recommended that Holter monitoring is used in patients with chest pain and suspected arrhythmias, and considered in patients with suspected vasospastic angina. It is not recommended as a routine investigation in patients with chronic coronary syndromes.
When to refer
Not all patients should be referred nor wish to be referred – often patients with chronic coronary syndromes are managed appropriately by their general practitioner.31 However, those in the following groups should be considered for early referral to a cardiologist:32,33
- new onset of chest pain suspected to be ischaemic, with
- exacerbation of stable angina or recurrence of old angina
- ECG evidence of previous MI/other abnormality
- previous MI, coronary artery bypass graft or percutaneous coronary intervention
- failure to respond to a maximum of two therapeutic doses of two anti-anginal drugs
- abnormal exercise tolerance test
- new concurrent atrial fibrillation, heart failure or ejection systolic murmur suggestive of aortic stenosis.
Some people with the following symptoms may have an ACS and should be considered for hospital admission.30 They include those with:
- Chest pain or pain in other areas (e.g. arms, back or jaw) lasting more than 15 minutes.
- Chest pain with nausea and vomiting, sweating, breathlessness or a combination
- Chest pain with haemodynamic instability
- New onset chest pain, or deterioration in previously stable angina, with frequent episodes on little or no exertion, and lasting longer than 15 minutes.
Patients with the above should be urgently transferred to hospital.
Key learning messages
- A detailed clinical assessment is critical in the work-up of chronic coronary syndromes
- Non-invasive functional testing or CTCA has been recommended as an initial test to diagnose myocardial ischaemic or CAD in chronic coronary syndromes (see Angina module 5: advanced cardiac imaging)34
close window and return to take test
References
- Press release: New NICE guidelines on diagnosis of chest pain set to save thousands of lives. London: National Institute for Health and Clinical Excellence, 2010.
- Ruigómez A, Rodriquez LAG, Wallander M-A et al. Chest pain in general practice: incidence, comorbidity and mortality. Fam Pract 2006;23:167–74. https://doi.org/10.1093/fampra/cmi124
- Erhardt L, Herlitz J, Bossaert L et al. Task force on the management of chest pain. Euro Heart J 2002;23:1153–76. http://dx.doi.org/10.1053/euhj.2002.3194
- Allamsetty S, Seepana S, Griffith KE. 10 steps before you refer for chest pain. Br J Cardiol 2009;16:80–4. https://bjcardio.co.uk/2009/03/10-steps-before-you-refer-for-chest-pain/
- Knuuti J, Wijns W, Saraste A et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology. Eur Heart J 2019 (published online 31st August 2019). https://doi.org/10.1093/eurheartj/ehz425
- National Institute for Health and Care Excellence (NICE). Chest pain of recent onset: Clinical guideline [CG95]. London: National Clinical Guideline Centre for Acute and Chronic Conditions, 2010. http://guidance.nice.org.uk/CG95
- Mieres JH, Gulati M, Merz N B et al. AHA consensus statement. Role of non-invasive testing in the clinical evaluation of women with suspected ischemic heart disease. Circulation 2014;130:350–79. http://dx.doi.org/10.1161/CIR.0000000000000061
- Dubrey SW, Ghonim S, Teoh M. Acute coronary syndromes among South Asian subgroups in the UK: symptoms and epidemiology. Br J Cardiol 2014;21:153–7. http://dx.doi.org/10.5837/bjc.2014.033
- Canadian Cardiovascular Society: (www.ccs.ca)
- Yelland MJ. Outpatient evaluation of the adult patient with chest pain.
https://www.uptodate.com/contents/outpatient-evaluation-of-the-adult-with-chest-pain?search=chest%20pain%20differential&source=search_result&selectedTitle=2~150&usage_type=default&display_rank=2 (last accessed 24th October 2019) - National Institute for Health and Care Excellence (NICE). NICE pathways: gastrointestinal tract (upper) cancers.
https://pathways.nice.org.uk/pathways/suspected-cancer-recognition-and-referral#path=view%3A/pathways/suspected-cancer-recognition-and-referral/suspected-cancer-recognition-and-referral-site-or-type-of-cancer.xml&content=view-node%3Anodes-gastrointestinal-tract-upper-cancers (last accessed 24th October 2019). - Schroeder K. Assessment of chest pain in primary care. InnovAiT 2008;1:8–14. http://dx.doi.org/10.1093/innovait/inm011
- National Institute for Health and Care Excellence. CVD risk assessment and management (revised March 2019).
https://cks.nice.org.uk/cvd-risk-assessment-and-management (last accessed 24th October 2019) - Daly C, Norrie J, Murdoch DL, Ford I, Dargie HJ, Fox K; TIBET (Total Ischaemic Burden European Trial) study group. The value of routine non-invasive tests to predict clinical outcome in stable angina. Eur Heart J 2003;24:532–40. https://dx.doi.org/10.1016/s0195-668x(02)00820-5
- Miller JM, Rochitte CE, Dewey M et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359:2324–36. https://doi.org/10.1056/NEJMoa0806576
- SCOT-HEART Investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383–91. https://dx.doi.org/10.1016/S0140-6736(15)60291-4
- Moss AJ, Williams MC, Newby DE, Nicol ED. The updated NICE guidelines: cardiac CT as the first-line test for coronary artery disease. Curr Cardiovasc Imaging Rep 2017;10(5):15. https://dx.doi.org/10.1007/s12410-017-9412-6
- Villines TC, Hulten EA, et al; CONFIRM Registry Investigators. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry. J Am Coll Cardiol 2011;58:2533–40. https://dx.doi.org/10.1016/j.jacc.2011.10.851
- Brady P, Kellon A, Hyde T et al. CT coronary angiography with HeartFlow®: a user’s perspective. Br J Cardiol 2019;26:105–9. https://dx.doi.org/10.5837/bjc.2019.029
- Knuuti J, Bengel F, Bax JJ et al. Risks and benefits of cardiac imaging: an analysis of risks related to imaging for coronary artery disease. Eur Heart J 2014;35:633–8. https://doi.org/10.1093/eurheartj/eht512
- Castellano IA, Nicol ED, Bull RK et al. A prospective national survey of coronary CT angiography radiation doses in the United Kingdom. J Cardiovasc Comput Tomogr 2017;11:268–73. https://dx.doi.org/10.1016/j.jcct.2017.05.002
- Smith-Bindman R, Lipson J, Marcus R et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009;169:2078–86. https://dx.doi.org/10.1001%2Farchinternmed.2009.427
- Faletra FF, Angeli ID, Kiersy C et al. Estimates of lifetime attributable risk of cancer after a single radiation exposure from 64-slice computed tomographic coronary angiography. Heart 2010;96:927-32. http://dx.doi.org/10.1136/hrt.2009.186973
- Knuuti J, Ballo H, Juarez-Orozco LE, et al. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: a meta-analysis focused on post-test disease probability. Eur Heart J 2018;39:3322–30. https://dx.doi.org/10.1093/eurheartj/ehy267
- Lubbers M, Dedic A, Coenen A, et al. Calcium imaging and selective computed tomography angiography in comparison to functional testing for suspected coronary artery disease: the multicentre, randomized CRESCENT trial. Eur Heart J 2016;37:1232–43. https://doi.org/10.1093/eurheartj/ehv700
- Zacharias K, Ahmed A, Shah BN, et al. Relative clinical and economic impact of exercise echocardiography vs. exercise electrocardiography, as first line investigation in patients without known coronary artery disease and new stable angina: a randomized prospective study. Eur Heart J Cardiovasc Imaging 2017;18:195–202. https://doi.org/10.1093/ehjci/jew049
- Sekhri N, Feder GS, Jumghans C et al. Incremental prognostic value of the exercise electrocardiogram in the initial assessment of patients with suspected angina: cohort study. BMJ 2008;337:a2240. https://dx.doi.org/10.1136/bmj.a2240
- Rajani R, Underwood SR. The exercise ECG – here today, gone tomorrow? Br J Cardiol 2011;18:7–8. https://bjcardio.co.uk/2011/02/the-exercise-ecg-here-today-gone-tomorrow/
- Wright C. Holter monitoring and the signal-averaged ECG in assessment of myocardial ischaemia. In: Purcell H, Kaddoura S (eds.). Angina: a systematic guide to investigation and treatment. Mosby, 2001.
- National Institute for Health and Care Excellence (NICE). NICE pathways. Assessment and immediate management of suspected acute coronary syndrome.
https://pathways.nice.org.uk/pathways/chest-pain#path=view%3A/pathways/chest-pain/assessment-and-immediate-management-of-suspected-acute-coronary-syndrome.xml&content=view-node%3Anodes-initial-assessment (last accessed 28th October 2019). - Gandhi MM, Lampe FC, Wood DA. Management of angina pectoris in general practice: a questionnaire survey of general practitioners. Br J Gen Pract 1995;45(390):11-3.
- Scottish Intercollegiate Guidelines Network (SIGN). Management of stable angina (SIGN publication number 151. Edinburgh: SIGN, April 2018.
https://www.sign.ac.uk/assets/sign151.pdf (last accessed 28th October 2019) - GP notebook. Referral criteria from primary care: stable angina. https://www.gpnotebook.co.uk/simplepage.cfm?ID=x20091201105452182440 (last accessed 28th October 2019).
- Fox KF. Coronary disease: investigation and management of chest pain. Heart 2005;91:105–10. http://dx.doi.org/10.1136/hrt.2003.031062


All rights reserved. No part of this programme may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publishers, Medinews (Cardiology) Limited.
It shall not, by way of trade or otherwise, be lent, re-sold, hired or otherwise circulated without the publisher’s prior consent.
Medical knowledge is constantly changing. As new information becomes available, changes in treatment, procedures, equipment and the use of drugs becomes necessary. The editors/authors/contributors and the publishers have taken care to ensure that the information given in this text is accurate and up to date. Readers are strongly advised to confirm that the information, especially with regard to drug usage, complies with the latest legislation and standards of practice.
Healthcare professionals should consult up-to-date Prescribing Information and the full Summary of Product Characteristics available from the manufacturers before prescribing any product. Medinews (Cardiology) Limited cannot accept responsibility for any errors in prescribing which may occur.