The renin–angiotensin system (RAS) has been at the forefront of research aimed at mitigating the infectivity and mortality associated with the coronavirus disease 2019 (COVID-19) pandemic. This stems from the observation that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the pathogen that causes COVID-19, utilises angiotensin-converting enzyme 2 (ACE2) as its receptor to invade host cells. Since emergence of COVID-19, conflicting guidance has been published on the use of medications that may increase ACE2 levels. Specifically, initial reports suggested that ACE inhibitors and angiotensin II type 1 receptor blockers (ARBs) may result in increased virulence of COVID-19 due to elevated ACE2. Thus, discontinuation of these RAS blockers was advised. However, the data on ACE2 expression with use of RAS blockers in humans without COVID-19 are not clear, and for humans with COVID-19 are not yet available. Moreover, discontinuation of these medications may be deleterious in some patients for whom they are prescribed to treat heart failure, hypertension and ischaemic heart disease. For this reason, professional organisations, including the American College of Cardiology, American Heart Association, Heart Failure Society of America and the European Society of Cardiology, have issued statements advising against discontinuation of any RAS-related treatments in patients during the COVID-19 crisis.
The issue regarding use of RAS blockers in the context of COVID-19 has previously been reviewed.1,2 Most recently, emerging data suggest no harm is associated with use of ACE inhibitors or ARBs in COVID-19.3,4 In this perspective, we discuss a related aspect that was first raised by Acanfora and colleagues,5 namely, the potential benefit of neprilysin inhibitors and their role in modulating levels of RAS components. Similar to the situation for ACE inhibitors and ARBs, it seems there are mixed opinions on the utility of neprilysin inhibitors in COVID-19. In a recent review, it was postulated that increasing neprilysin activity might mitigate COVID-19 pathogenesis.6 Here, we present a case that is in line with arguments made by Acanfora et al.;5 that is, in addition to anti-inflammatory effects, we posit that neprilysin inhibition may augment levels of the RAS metabolite, angiotensin(1–7), which could improve outcomes of SARS-CoV-2 infection.
Neprilysin inhibitors
Although neprilysin inhibitors have been used for decades to treat acute diarrhoea, to our knowledge, there have been no studies to evaluate their impact on angiotensin(1–7) levels in humans. Recently, sacubitril/valsartan, the first-in-class angiotensin receptor/neprilysin inhibitor (ARNI), was developed for the treatment of heart failure with reduced ejection fraction. Initially, it was shown to be superior to the ACE inhibitor, enalapril, in reducing rates of cardiovascular death and hospitalisation. Later, studies of sacubitril/valsartan demonstrated antihypertensive and nephroprotective properties, as well as improved glycaemic control in diabetic patients. Sacubitril inhibits neprilysin, which is a peptidase of the RAS capable of both generating and hydrolysing angiotensin(1–7) (figure 1).
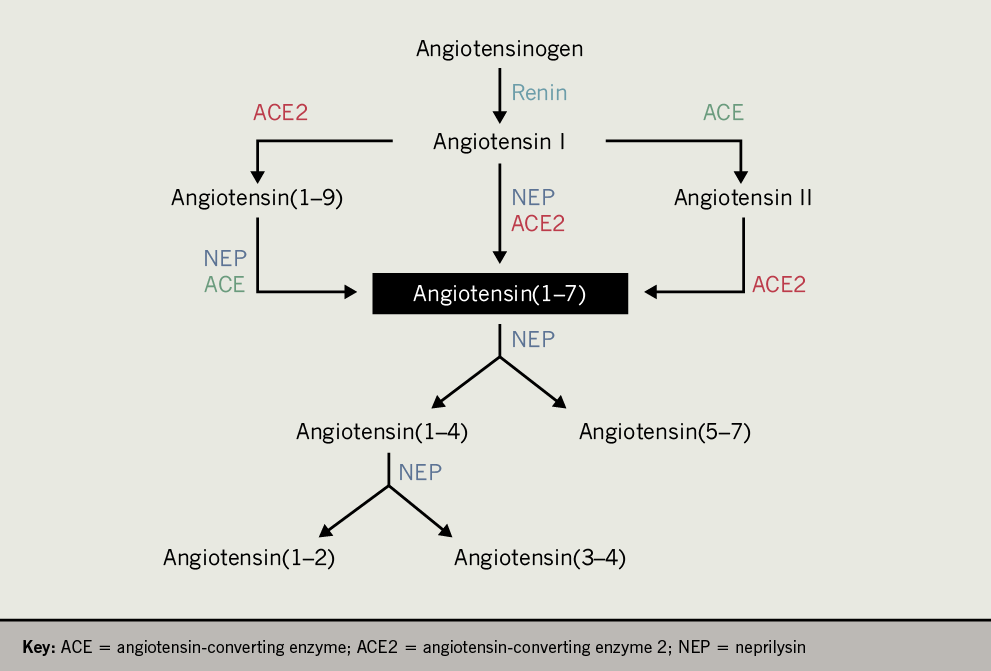
Angiotensin(1–7) binds the G protein-coupled receptor MasR to elicit responses that can counteract those of angiotensin II. Generation of angiotensin(1–7) has typically been thought to occur via ACE2-mediated cleavage of angiotensin I or angiotensin II, or ACE-mediated cleavage of angiotensin(1–9) (figure 1). Importantly, neprilysin is also capable of generating angiotensin(1–7) by cleaving angiotensin I and angiotensin (1–9) (figure 1). However, angiotensin(1–7) is further cleaved by neprilysin into several smaller peptides.7 Thus, inhibition of neprilysin may favour preservation of angiotensin(1–7), even in the face of reduced neprilysin-mediated generation of angiotensin(1–7), since there is redundancy in the RAS to allow for ACE and/or ACE2 to generate angiotensin(1–7).
In support of our proposed hypothesis, our unpublished observations in neprilysin-deficient mice show that plasma angiotensin(1–7) levels are elevated 1.9 ± 0.3 fold when compared with wild-type mice under conditions of increased dietary fat (p<0.05, n=3–5). This elevation in circulating angiotensin(1–7) is associated with improved glucose homeostasis,8 which may be advantageous in COVID-19 patients with diabetes. Thus, we propose that medications which inhibit neprilysin and increase ACE2 activity, such as ARNI, may elicit beneficial effects in COVID-19 patients by augmenting angiotensin(1–7) levels.
Angiotensin(1–7) and comorbidities
In addition to manifestation of severe pulmonary disease, the most common comorbidities that confer increased risk for hospitalisation and mortality of the COVID-19 infection are hypertension, cardiovascular disease and diabetes.9 In COVID-19 patients with these co-existing conditions, angiotensin(1–7) may prove particularly beneficial as it has been shown to reduce lung inflammation, fibrosis and pulmonary arterial hypertension.10,11 Additionally, it is cardioprotective, having both anti-arrhythmogenic and anti-hypertrophic effects, and inducing vasodilation in hypertensive conditions.10
In diabetes, angiotensin(1–7) has multiple actions that improve glucose homeostasis and reduce diabetic complications. For example, it modulates insulin-signalling pathways in liver, skeletal muscle and adipose tissue, and enhances endocrine pancreas function by regulating blood flow and reducing apoptosis of endothelial cells.10 The net effect is enhanced glucose tolerance, insulin sensitivity and insulin secretion.
Angiotensin(1–7) also protects against the development of vascular complications by inhibiting vascular inflammation and proliferation, and ameliorating endothelial dysfunction.10 Together, these actions of angiotensin(1–7) may result in clinical benefits that have the potential to reduce severe outcomes of COVID-19 infection in high-risk patients. Moreover, such benefits would argue against discontinuation of medications that increase angiotensin(1–7) levels, particularly those that include neprilysin inhibitors.
Other pathways
It is noteworthy to also emphasise that neprilysin inhibition per se can modulate pathways that are independent of the RAS. This is mediated by neprilysin substrates whose degradation is inhibited, some of which may protect against lung injury, cardiovascular disease and/or diabetes. For example, natriuretic peptides and glucagon-like peptide-1 are neprilysin substrates with pleiotropic protective effects. Augmenting levels of such substrates by continuing ARNI treatment may improve underlying conditions that otherwise could exacerbate outcomes of COVID-19 infection. In considering such improvements, and that many neprilysin substrates have anti-inflammatory properties, it may be difficult to dissociate the benefits of ARNI to reduce blood pressure or glucose levels from the action of angiotensin(1–7) to reduce inflammation. Perhaps studies in preclinical models would help elucidate the mechanism(s) by which neprilysin substrates, besides angiotensin(1–7), may confer benefit in COVID-19.
Proposal
In conclusion, the possibility that dual neprilysin inhibition and angiotensin II receptor blockade could significantly augment levels of the protective peptide angiotensin(1–7), would argue that high-risk patients may benefit from combination medications like sacubitril/valsartan. We propose that this be considered in the treatment of COVID-19 patients with co-existing conditions, and that analysis of clinical records be undertaken to inform whether the prognosis of patients treated with sacubitril/valsartan is superior to those treated with alternative RAS blockers or other standard therapies for cardiovascular disease. The latter may require large population studies from combined datasets, given that an initial report, with a relatively small sample size, indicates the proportion of COVID-19 patients treated with sacubitril/valsartan versus other RAS blockers is low.3 To complement such records analyses, preclinical studies may inform on the potential benefits of dual neprilysin inhibition and angiotensin II receptor blockade in COVID-19.
Conflicts of Interest
SZ has previously received research support from Novartis Pharmaceuticals Corporation for preclinical studies. NE has no conflicts of interest to disclose.
Funding
The authors are supported by the Department of Veterans Affairs, VA Puget Sound Health Care System (Seattle, WA, USA), Seattle Institute for Biomedical and Clinical Research (Seattle, WA, USA) and National Institutes of Health (grants R01DK098506 and P30DK017047). NE is supported by the Dick and Julia McAbee Endowed Postdoctoral Fellowship in Diabetes from the University of Washington.
Study approval
Animal studies were approved by the Veterans Affairs Puget Sound Health Care System Institutional Animal Care and Use Committee in Seattle, WA, USA.
Acknowledgements
We thank S E Kahn and R L Hull (Department of Medicine, University of Washington) for valuable discussions and feedback during the writing of this manuscript. We thank B Barrow, D Hackney, S Mongovin and C Schmidt from Seattle Institute for Biomedical and Clinical Research, USA, for technical support in generating the plasma angiotensin(1–7) data.
References
1. Patel AB, Verma A. COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA 2020;online first. https://doi.org/10.1001/jama.2020.4812
2. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020;382:1653–9. https://doi.org/10.1056/NEJMsr2005760
3. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med 2020;382:2431–40. https://doi.org/10.1056/NEJMoa2006923
4. Reynolds HR, Adhikari S, Pulgarin C et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med 2020;382:2441–8. https://doi.org/10.1056/NEJMoa2008975
5. Acanfora D, Ciccone MM, Scicchitano P, Acanfora C, Casucci G. Neprilysin inhibitor-angiotensin II receptor blocker combination (sacubitril/valsartan): rationale for adoption in SARS-CoV-2 patients. Eur Heart J Cardiovasc Pharmacother 2020;6:135–6. https://doi.org/10.1093/ehjcvp/pvaa028
6. Mohammed El Tabaa M, Mohammed El Tabaa M. Targeting neprilysin (NEP) pathways: a potential new hope to defeat COVID-19 ghost. Biochem Pharmacol 2020;178:114057. https://doi.org/10.1016/j.bcp.2020.114057
7. Brar GS, Barrow BM, Watson M et al. Neprilysin is required for angiotensin-(1-7)’s ability to enhance insulin secretion via its proteolytic activity to generate angiotensin-(1-2). Diabetes 2017;66:2201–12. https://doi.org/10.2337/db16-1318
8. Willard JR, Barrow BM, Zraika S. Improved glycaemia in high-fat-fed neprilysin-deficient mice is associated with reduced DPP-4 activity and increased active GLP-1 levels. Diabetologia 2017;60:701–08. https://doi.org/10.1007/s00125-016-4172-4
9. Wang D, Hu B, Hu C et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. https://doi.org/10.1001/jama.2020.1585
10. Santos RAS, Sampaio WO, Alzamora AC et al. The ACE2/Angiotensin-(1-7)/MAS axis of the renin-angiotensin system: focus on angiotensin-(1-7). Physiol Rev 2018;98:505–53. https://doi.org/10.1152/physrev.00023.2016
11. Peiro C, Moncada S. Substituting angiotensin-(1-7) to prevent lung damage in SARS-CoV-2 infection? Circulation 2020;141:1665–6. https://doi.org/10.1161/CIRCULATIONAHA.120.047297
