Radial artery access has transformed cardiac catheterisation, allowing it to be performed in a daycase setting, saving both hospital beds, and nursing care costs. However, there are two common and seemingly diametrically opposite complications. These are radial artery occlusion and forearm haematoma; the former could be reduced by heparin, but at the expense of precipitating the latter. These complications increase proportionally to the size of radial artery sheath used. Interestingly, by cannulating the radial artery more distally beyond its bifurcation in the hand, the distal radial approach appears to be the ‘one stone, two birds’ or the synchronous Chinese idiom, ‘yīshí’èrniǎo’s’ solution, reducing both complications at the same time. Extending this further and downsizing to a 4Fr catheter system, heparin use could be spared altogether, without complications, and haemostasis achieved with short manual pressure at the puncture site. Hence, further cost savings by foregoing commercial compression bands, and abolishing access site care for nurses. We illustrate the above strategy in a patient with challenging radial anatomy, made simple and easy.
Introduction
Giving heparin with radial artery access cardiac catheterisation is standard practice to prevent radial artery occlusion (RAO).1,2 However, this is complicated by access-site bleeding in nearly one quarter of cases,2,3 and more significant forearm haematoma approaching a 10% incidence with the 6Fr catheter system.4 The ‘newer’ distal radial artery (dRA) approach, where the radial artery is punctured in the anatomical ‘snuff box’, i.e. beyond the radial artery bifurcation into the palmar arch branches, has significantly less RAO.5 Hence, we have defaulted to the dRA approach in our allcomers’ cardiac catheterisation practice over the last three years.6 Going against the grain, in our experience, the combination of the dRA approach and the 4Fr catheter system allows heparin to be omitted completely, simplifying diagnostic coronary angiography, as described in our illustrative case below.
Case
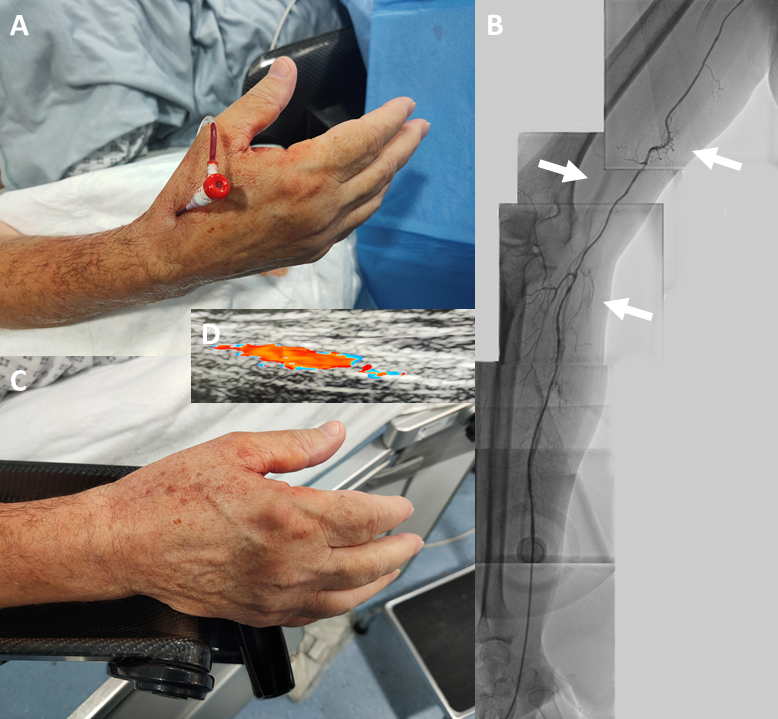
A 70-year-old man was admitted for a daycase diagnostic coronary angiogram as part of the work-up for his severe aortic stenosis management, as he had become symptomatic with postural dizziness, chest pain and breathlessness. His right dRA was cannulated with a 4Fr Prelude IDeal 11 cm sheath (outside diameter 1.78 mm vs. 2.62 mm for a 6Fr sheath) (Merit Medical, South Jordan, UT, USA) (figure 1A). Following 5 mg of intra-arterial verapamil, a JR4 4Fr catheter was advanced into the aortic root with a J-tipped 0.035″ wire, and the right coronary artery was imaged. Next, the same wire was used to exchange for a JL3.5 4Fr catheter.
The left coronary artery was intubated, and angiograms obtained, but there appeared to be some resistance in torquing the catheter, with the presumption that there might be radial artery spasm. A radial arteriogram was therefore undertaken (figure 1B), and this shows a high take off small and tortuous radial ‘recurrent’ artery arising from the axillary artery, which then joins the radial artery loop before continuing as the radial artery. This explains the aforementioned ‘catheter friction’. The sheath was removed, and manual pressure was applied to the puncture site for 10 minutes, which achieved haemostasis (figure 1C). The radial pulse remains bounding, and a vascular Doppler ultrasound confirms a widely patent radial artery with normal flow pattern (figure 1D).
Discussion
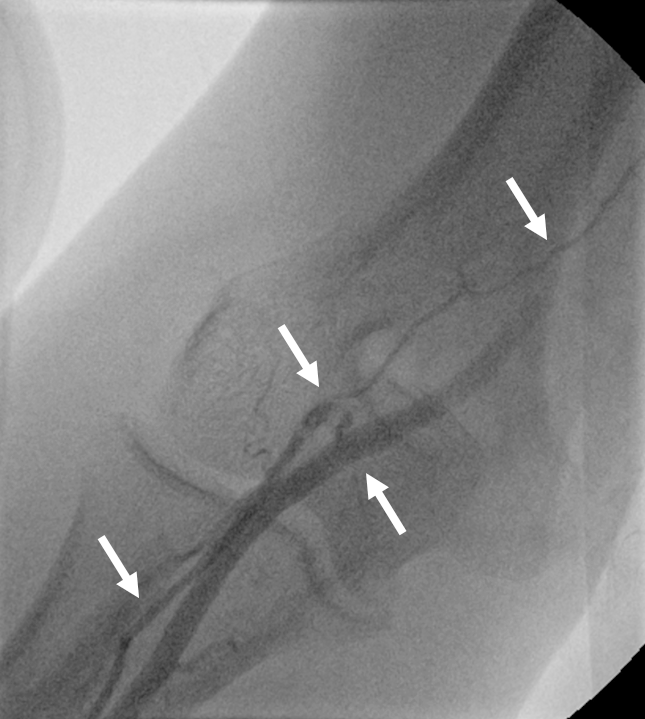
The radial artery approach is now the recommended access for cardiac catheterisation, although it has a roughly 10% conversion rate due to failure to cannulate the artery, radial and subclavian artery anatomical challenges or difficulty in engaging the coronary ostiae, therefore, necessitating an alternative route.7 There are, however, mitigating factors and techniques that potentially could reduce this to under 1% for the diehard radialists.6,8-11 The above described case is one such example, the very small, tortuous and high take off radial recurrent artery is not usually accessible to the commonly used 5Fr and 6Fr catheters, and if brute force is applied could lead to catheter entrapment, radial artery avulsion or bleeding complications.12 This anatomical variant is also poorly recognised,7 and figure 2 shows a contrasting, typical and generic case where procedural failure occurred requiring access site conversion. With a default 4Fr catheter system, however, this challenging anatomy would have been missed had there not been ‘slight’ resistance in turning the catheter followed by a radial arteriogram to determine the cause.
Radial artery access permits cardiac catheterisation to be safely undertaken in the ambulatory setting in the radial lounge, which is a significant service improvement in freeing up hospital beds. There is also one major drawback to this access, that is, despite routine heparin use, RAO occurs in 5–10% of cases.2 There is evidence that giving even higher doses of heparin (100 IU/kg vs. 50 IU/kg) reduces this complication, but at the expense of a one-in-four chance of forearm haematoma developing, regardless of dosing, perhaps explained by the high rate of attempted patent haemostasis in this study to avoid RAO.2 On the other hand, data are emerging to suggest that the RAO rate with the dRA approach is well under 1%.5 The reason for this is because the puncture site is beyond the radial artery bifurcation. Hence, it is naturally patent haemostatic when the access site is compressed, as blood flow continues in the radial artery into the superficial palmar arch branch. Another important factor for RAO is radial artery injury by the sheath, i.e. the bigger the sheath, the higher the RAO rate.13 Taking both into account, over the last three years we routinely performed our diagnostic coronary angiograms via dRA with a 4Fr catheter system, and without heparin.6 We believe that the patent haemostasis afforded by the dRA approach makes heparin superfluous, unless it is given for coronary intervention, for 5Fr and 6Fr catheter systems.
In conclusion, there are three-fold benefits with our technique: first, haemostasis could be achieved with short manual pressure without need for a dedicated commercial compression band, yet no appreciable RAO or haematoma, hence, cost savings; second, post-procedural management is simplified for nurses, saving them time from active access site care; and third, with 4Fr or even 5Fr catheter, coronary angiography is ‘atraumatic’ and the slender catheter navigates with ease the small and adverse anatomies, as exemplified by this case, without compromising the quality of the coronary angiogram, and there is no barrier to upsize the sheath if it is expedient to do so, easily up to a 7Fr catheter system for complex coronary interventional procedures.6,10
Key messages
- The distal radial artery approach to cardiac catheterisation has all the benefits of radial artery access, in addition to a radial artery occlusion rate of under 1%, compared with 5–10%
- Downsizing to a 4Fr catheter system makes heparin sparing possible, allowing rapid haemostasis of the puncture site with manual pressure alone
- The 4Fr catheter system also allows event-free cardiac catheterisation when tortuous and small conduits are encountered
Conflicts of interest
None declared.
Funding
None.
Acknowledgements
We thank the Department of Cardiology as led by Dr Rajan Sharma, Cardiac Catheter Laboratory Leaders: Mary Keal (Matron), Alexander Grimster (Head of Cardiac Physiology), and Dinesh Sajnani (Head of Cardiac Radiography), the patient described in this case who placed his complete faith and trust in us to look after him, Dr Kayla Chiew for proofreading, and last but not least the Departmental and Cardiac Catheter Laboratory auxillary and nursing staff involved in the care of this patient.
Patient consent
The patient has given informed consent for his case to be shared for learned communication.
References
1. Bernat I, Aminian A, Pancholy S et al. Best practices for the prevention of radial artery occlusion after transradial diagnostic angiography and intervention: an International Consensus Paper. JACC Cardiovasc Interv 2019;12:2235–46. https://doi.org/10.1016/j.jcin.2019.07.043
2. Hahalis GN, Leopoulou M, Tsigkas G et al. Multicenter randomized evaluation of high versus standard heparin dose on incident radial arterial occlusion after transradial coronary angiography: the SPIRIT OF ARTEMIS study. JACC Cardiovasc Interv 2018;11:2241–50. https://doi.org/10.1016/j.jcin.2018.08.009
3. Sandoval Y, Bell MR, Gulati R. Transradial artery access complications. Circ Cardiovasc Interv 2019;12:e007386. https://doi.org/10.1161/CIRCINTERVENTIONS.119.007386
4. Garg N, Umamaheswar KL, Kapoor A et al. Incidence and predictors of forearm hematoma during the transradial approach for percutaneous coronary interventions. Indian Heart J 2019;71:136–42. https://doi.org/10.1016/j.ihj.2019.04.014
5. Eid-Lidt G, Rivera Rodriguez A, Jimenez Castellanos J, Farjat Pasos JI, Estrada Lopez KE, Gaspar J. Distal radial artery approach to prevent radial artery occlusion trial. JACC Cardiovasc Interv 2021;14:378–85. https://doi.org/10.1016/j.jcin.2020.10.013
6. Ferreira-Martins J, Lim PO. Two-step distal radial artery cannulation for challenging radial anatomies. Cardiology 2021;146:144–50. https://doi.org/10.1159/000510293
7. Mansour S, Mohamad A, Ibrahim Y et al. Challenge to reduce crossover from radial to femoral access for coronary procedures: “RURU” approach. A single center, single operator experience in 1000 procedures. J Cardiovasc Dis Diagn 2019;7:362. Available from: https://www.hilarispublisher.com/open-access/challenge-to-reduce-crossover-from-radial-to-femoral-access-for-coronary-procedures-ldquorururdquo-approach-a-single-cen.pdf
8. Li Kam Wa M, Lim PO. A simple technique for IMA graft angiography and PCI using contralateral radial access. Br J Cardiol 2019;26:110–13. https://doi.org/10.5837/bjc.2019.024
9. Lim PO, Dzavik V. Balloon crush: treatment of bifurcation lesions using the crush stenting technique as adapted for transradial approach of percutaneous coronary intervention. Catheter Cardiovasc Interv 2004;63:412–16. https://doi.org/10.1002/ccd.20179
10. Manoharan K, Lim PO. Overcoming the 360 degree radial artery loop. Eurointervention 2021:[online first 17 March 2021]. Available from: https://www.pcronline.com/Cases-resources-images/Images-interventional-cardiology/EuroIntervention-images/Overcoming-the-360-degree-radial-artery-loop
11. Manoharan K, Lim PO. Distal radial approach to reaccess and recanalize the occluded radial artery. Eurointervention 2021:[online first 30 April 2021]. Available from: https://www.pcronline.com/Cases-resources-images/Images-interventional-cardiology/EuroIntervention-images/Distal-radial-approach-reaccess-recanalize-occluded-radial-artery
12. Alkhouli M, Cohen HA, Bashir R. Radial artery avulsion – a rare complication of transradial catheterization. Catheter Cardiovasc Interv 2015;85:E32–E34. https://doi.org/10.1002/ccd.25528
13. Saito S, Ikei H, Hosokawa G, Tanaka S. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Catheter Cardiovasc Interv 1999;46:173–8.
