Cardiac magnetic resonance (CMR) has much to offer in the clinical assessment of intra-cardiac space-occupying lesions (SOL). Below we describe the use of CMR as a second-line investigation complementing the use of other imaging modalities, using the example of three patients with atrial SOL. We briefly review the literature and discuss the use of CMR within the context of multi-modality imaging of cardiac SOL.
Case 1
A 49-year-old woman with an unremarkable past medical history presented to her local hospital with irregular palpitations and two syncopal episodes. On both occasions she had regained consciousness without any neurological features, neither as prodrome nor in recovery. Examination revealed a diastolic murmur. Electrocardiogram (ECG) and chest X-ray were normal.
A transthoracic echocardiogram (TTE) revealed a 2–3 cm mass in a non-dilated left atrium. Her transoesophageal echocardiogram showed the mass to be located close to the right, lower pulmonary vein but suggested the point of attachment to be the posterior wall rather than the atrial septum, somewhat atypical for atrial myxoma. Although she had a normal-sized atrium, paroxysmal atrial fibrillation was suspected from her history and, consequently, a differential diagnosis of thrombus was entertained, given that atrial thrombus is per se a more frequent finding than atrial myxoma,1 and tends to arise from the posterior wall, rather than atrial septum.2
A myocardial contrast enhancement echo was performed to demonstrate perfusion. Here, transpulmonary contrast is administered using low-energy ultrasound interleaved with high-energy pulses to destroy the phospholipid-shell bubbles within the field of view and monitoring of tissue bubble replenishment to assess perfusion. This supported a diagnosis of a cardiac tumour over thrombus.
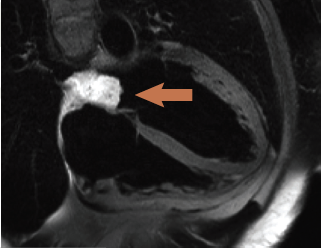
Cardiac magnetic resonance (CMR) (figure 1) confirmed a 3.7 x 2.3 x 2 cm mass with a broad-based attachment to the inter-atrial septum at the floor of the fossa ovalis, partially obstructing the right, lower pulmonary vein. The mass had high signal intensity on T2-weighted spin echo images (figure 1), persisting on fat suppression imaging, excluding a lipoma or liposarcoma. Signal intensity doubled on first-pass perfusion, excluding thrombus. Delayed enhancement imaging revealed patchy hyperenhancement suggestive of cystic cavitation, features all strongly suggestive of a myxoma. The patient proceeded to an uncomplicated surgical removal of the mass. Histology revealed a gelatinous mass with a chronic inflammatory cell infiltrate at the periphery of the tumour. No necrosis was seen within the mass.
Case 2
A 59-year-old woman presented to a nearby hospital with two days of left iliac fossa pain. On admission she had a low-grade fever, but other observations were normal, as was the remainder of the clinical examination. All blood tests were normal except for raised C-reactive protein (CRP) and white cell count (WCC). ECG and plain X-rays were unremarkable. Diverticulitis was initially suspected.
A contrast-enhanced computed tomography (CT) scan revealed multiple splenic infarcts. Additionally, a filling defect was noticed in a dilated left atrium. A subsequent TTE was performed showing a homogenous left atrial mass prolapsing into the mitral valve plane. The point of attachment was thought to be the inter-atrial septum but this was not clearly seen.
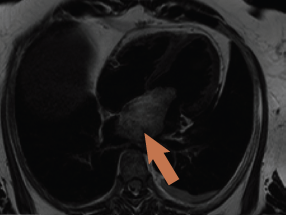
CMR was again requested to tissue characterise the mass and delineate the site of attachment in relation to the neighbouring structures. The scan confirmed a 7 x 3.6 x 3.7 cm mass in the left atrium attached to the fossa ovalis via a stalk, with a 1.3 cm footprint (figure 2). The mass had low signal intensity on ‘white blood’ imaging using a steady-state free precession sequence (True-FISP), with a similar isodense signal to myocardium on T1 imaging. The mass was signal intense on T2-weighted images, and remained bright on fat-suppression with no significant contrast uptake on first-pass perfusion. There was no hyperenhancement on delayed enhancement imaging.
Surgical removal and histological assessment confirmed atrial myxoma attached to the superior and posterior aspects of the left atrial side of the fossa ovalis. Histological assessment demonstrated a haemorrhagic mass with haemosiderin-laden macrophages.
Case 3
A 42-year-old man presented with abdominal pain. He was found to have an iron-deficiency anaemia with a family history of bowel cancer. He, therefore, went on to have excision of a polyp by colonoscopy, which also incidentally identified an abnormal anal tag, subsequently found to be a squamous cell carcinoma on histology; this was also excised. A contrast CT scan of the abdomen was arranged due to ongoing pain, and this revealed an area of thickened, inflamed large bowel loop, thought to be due to an embolic event. A filling defect in the left atrium was also described. The appearances of the mass on subsequent echocardiography were suggestive of a myxoma; a small pericardial effusion was also seen.
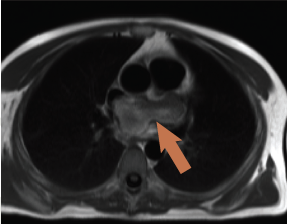
CMR was performed revealing a multi-lobular mass of approximately 3.8 x 4.2 x 3.5 cm (figure 3), thought to arise from the inferior aspect of the fossa ovalis extending with a broad base to the roof of the fossa, one lobe apparently growing into and almost completely filling the left atrial appendage (3 x 3.1 cm). There was partial obstruction to the lower pulmonary veins and compression of the superior vena cava/right atrial (SVC/RA) junction. On additional coronal cine imaging (figure 4), the mass appeared to be superior to the left atrial roof, and it was unclear if the mass had grown through the atrial roof or indeed primarily arisen from the left atrial roof and grown bi-directionally. The mass was mildly hyperintense on T1 and more so on T2, with evidence of cystic cavitation. First-pass perfusion demonstrated very little blood supply and a small pericardial effusion was also noted.
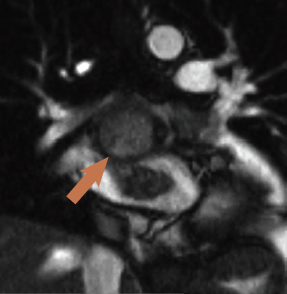
There was debate among cross-sectional imaging physicians about whether this was a myxoma or the anatomic appearance was supporting malignancy. The patient went for surgery where the mass was subtotally excised due to extension into the visceral pericardium and transverse sinus. Such behaviour is atypical for myxomas. Histology revealed myxoid stroma with considerable mitotic features, extensive necrosis and haphazard arrangement within myxoid stroma, features all in keeping with a grade 3 undifferentiated, pleomorphic sarcoma.
Discussion
Epidemiology of myxomas
Atrial myxomas are the most common benign, primary cardiac tumour (followed by lipomas and papillary fibroelastomas) and can vary in size from 1 to 15 cm.3 Approximately 80% are located in the left atrium, 13% right atrium and 1.3% are bi-atrial. Occurrence in the ventricles and on valves is described, but remains very rare.3-6
Although benign in terms of invasive potential, malignant haemodynamic features include valve obstruction, valvular regurgitation and systemic embolic phenomena.5 However, other malignant features include its ability to recur following resection, believed to be due to the multi-centric nature of the disease.7 The Carney syndrome accounts for 5–7% of cardiac myxomas and exhibits an autosomal dominant inheritance pattern, characterised by myxomas in cardiac and extracardiac sites associated with skin pigmentation and endocrine hyperactivity; thyroid and pituitary tumours are also associated.6,8
Modes of presentation
These can be divided into obstructive, embolic and constitutional. In a study of 112 patients,4 approximately 29% had evidence of systemic embolisation and 34% had constitutional symptoms thought to be due to elevated interluekin-6 (IL-6) levels.5 Another retrospective analysis of modes of presentation3 revealed around 15% present with chest pain and 19% present with arrhythmias in association with one of the above; 5% were asymptomatic and discovered on routine echocardiography performed for other reasons. The classic tumour ‘plop’ in diastole was identified in only a minority (15%) of cases.
Comparison of cases
All the cases reflect that ECG and chest X-ray are not helpful in the diagnosis. The ‘malignant’ potential of this benign tumour is emphasised by case 2 as is the poor negative predictive value of the tumour plop, which was clinically not detected on auscultation despite CMR evidence of tumour prolapse through the mitral valve.
Presentation with syncope has generally been considered to result from obstruction of mitral valve inflow. However, case 1 presented with syncope, yet cine imaging did not provide strong support for mitral valve inflow obstruction as the underlying mechanism. Indeed, this tumour was the smallest of the presented cases, with the least imaging evidence of impact on mitral valve inflow.
Case 3 is of particular interest as many of the imaging features, for example location and apparent origin, T1 and T2 signal characteristics, moderate signal increase on first-pass perfusion and diffuse hyperenhancement on late enhancement imaging, appeared in keeping with left atrial myxoma. Indeed, histology confirmed myxoid stroma not dissimilar from that seen in myxomas. However, some features, most notably the expansive and irregular (‘cauliflower’) growth, apparent growth into left atrial appendage (intra-operatively the left atrial appendage was found to be free of tumour mass but instead was completely externally compressed by the mass), compression of the SVC/RA junction, and the apparent invasion and penetration of the left atrial roof, were features pointing towards a possible malignancy. Somewhat ambivalent features were a small pericardial effusion and minimal mediastinal lymphadenopathy.
Multi-modality imaging
A range of imaging modalities were used in our cases. All had a TTE as this is the most widely available and versatile modality. TTE provides rapid anatomical and functional information and the classic pedunculated tumour with a stalk attaching it to the atrial septum is occasionally sufficient for diagnosis. Agostini et al. combined positron emission tomography (PET) imaging to demonstrate metabolic activity of the mass, but this has not found widespread clinical use.9
Contrast-enhanced CT characteristically reveals a lobular contour with lower or equivalent signal intensity than myocardium and allows accurate delineation of the point of attachment in some cases, but this becomes increasingly limited as the size of the tumour increases and obliterates the atrium. The detection of calcium or calcification is the domain of CT, which is occasionally helpful in the differential diagnosis of cardiac tumours from mitral annular calcification that can mimic tumour masses.10,11 Some early work suggested that mean CT signal intensity, as per Hounsfield Units (HU), could help differentiate myxomas from thrombus (mean value 44.5 HU for thrombus versus 30.2 HU for myxomas),10 but this has not been assessed in a larger case series.
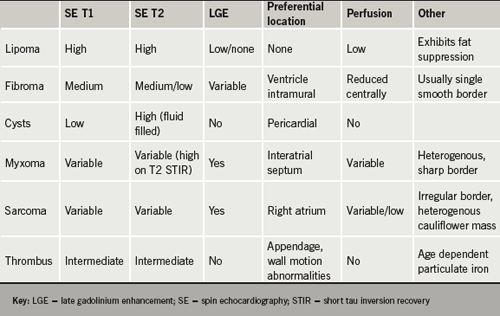
Advantages of CMR are its unparalleled soft-tissue imaging capabilities and, thus, its ability to tissue characterise by virtue of perfusion, late-enhancement imaging, fat-suppression and T1 and T2 characteristics.2,12 These are summarised in table 1.
Due to slice selectivity and reproducibility, first-pass perfusion is more reliably assessed on CMR than echocardiography. In fact, myocardial contrast echocardiography (MCE) can create false-positive results by virtue of the blood pool projecting onto the structure being interrogated (i.e. in front of the mass), giving the erroneous appearance of perfusion of the mass on a two-dimensional image. First-pass perfusion with CMR, if planned meticulously, avoids this problem.
Disadvantages of CMR and CT are the decline in image quality in the setting of frequent ectopic beats and irregular rhythms. Small, mobile, pedunculated myxomas can occasionally be overlooked during steady-state free precession imaging due to their alternating position throughout the cardiac cycle.15-17
Conclusion
From a practical point of view, it is possibly fair to say that the better image quality afforded by CMR imaging of larger cardiac masses outweigh the theoretical advantage, in terms of better temporal and spatial resolution, provided by TTE, in aiding the diagnosis. Morphological appearance and adherence to anatomical boarders are clinically more useful and reproducible features than signal intensity on T1 and T2 weighting. This is likely due to the histological overlap and non-uniformity of myxomas and malignant intra-cardiac space-occupying lesions.18
The above cases illustrate that myxomas display a broad spectrum of presenting clinical signs and symptoms, and a variety of imaging and histological findings. It is for this reason that a multi-modality imaging approach may be justified in selected cases.
While information obtained from such investigations will inform about the need, urgency and possible success of (complete) surgical removal, the ultimate diagnosis, however, must remain a histological one.
Conflict of interest
None declared.
Key messages
- Cardiac magnetic resonance is a powerful tool for the assessment of intra-cardiac masses, particularly in patients with sinus rhythm
- Morphological appearance of the space-occupying lesions and adherence to anatomical borders are clinically more important and useful features than signal intensity on T1 and T2 weighting
References
1. Matsuoka H, Hamada M, Honda T et al. Morphologic and histologic characterisation of cardiac myxomas by magnetic resonance imaging. Angiology 1996;47:693–8.
2. Sparrow PJ, Kurian JB, Jones TR, Sivananthan MU. MR imaging of cardiac tumours. Radiographics 2005;25:1255–76.
3. Grebenc ML, Rosado-de-Christenson ML, Green CE, Burke AP, Galvin JR. Cardiac myxoma: imaging features in 83 patients. Radiographics 2002;22:673–89.
4. Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore) 2001;80:159.
5. McManus B, Lee C. Primary tumours of the heart. In: Braunwald E (ed.). Heart disease. 8th edition. Philadelphia: Elsevier, 2008:1815–23.
6. Sheppard MN. Tumours of the heart. In: Camm J (ed.). The ESC textbook of cardiovascular medicine. Oxford: Oxford University Press, 2006:535–51.
7. Sadeghi N, Sadeghi S, Karimi A. Mitral valve recurrence of a left atrial myxoma. Eur J Cardiothorac Surg 2002;21:568–73.
8. Stratakis CA, Jenkins RB, Pras E et al. Cytogenetic and microsatellite alterations in tumors from patients with the syndrome of myxomas, spotty skin pigmentation, and endocrine overactivity (Carney complex). J Clin Endocrinol Metab 1996;81:3607–14.
9. Agostini D, Babatasi G, Galateau F et al. Detection of cardiac myxoma by F-18 FDG PET. Clin Nucl Med 1999;24:159.
10. Araoz PA, Mulvagh Sl, Tazelaar HD, Julsrud PR, Breen JF. CT and MR imaging of benign cardiac neoplasms with echocardiographic correlation. Radiographics 2000;20:1303–19.
11. Hongo M, Okubo S, Amemiya H et al. Diagnosis of left atrial masses by computed tomography: with special reference to the differentiation between mural thrombi and myxomas. J Cardiogr 1983;13:935–47.
12. Gilkeson RC, Chiles C. MR evaluation of cardiac and pericardial malignancy. Magn Reson Imaging Clin N Am 2003;11:173–86.
13. Lombardi M Frank H. Tumours and masses of the heart and pericardium. In: Schwitter J (ed.). CMR update. Zurich: Juerg Schwitter, 2008:190–206.
14. Bogaert J Dymarkowski S. Cardiac masses. In: Bogaert J (ed.). Clinical Cardiac MRI. Berlin: Springer, 2005:305–45.
15. Constantine G, Shan K, Flamm SD, Sivananthan MU. Role of MRI in clinical cardiology. Lancet 2004;363:2162–71.
16. Kaminaga T, Takeshita T, Kimura I. Role of magnetic resonance imaging for evaluation of tumors in the cardiac region. Eur Radiol 2003;13(suppl 4):L1–L10.
17. Luna A, Ribes R, Caro P, Vida J, Erasmus JJ. Evaluation of cardiac tumours with MRI. Eur Radiol 2005;15:1446–55.
18. Paydarfar D, Krieger D, Dib N et al. In vivo MRI and surgical histopathology of intracardiac masses: distinct features of subacute thrombi. Cardiology 2001;95:40–7.
