Renal artery stenosis is a condition that has significant effects on the progression and outcomes of co-existent cardiac disease. The most important cause of renal artery stenosis is atherosclerotic renovascular disease (ARVD). As the drugs and techniques used to manage ARVD are similar to those used to treat coronary artery disease, cardiologists are increasingly becoming involved in its management. However, while there are similarities, there are also significant differences in the management of ARVD and coronary artery disease. There are also many differing opinions on the best management. This review maps the minefield of conflicting evidence and gives clear, pragmatic guidelines regarding the management of patients with cardiorenal disease.
Introduction
Renal artery stenosis (RAS), traditionally the preserve of the nephrologist, is a condition of increasing interest to the cardiologist. Ninety per cent of RAS is caused by atherosclerosis and the risk factors for renal atherosclerosis and coronary atherosclerosis are the same. Furthermore, the presence of RAS alters the prognosis of co-existent cardiac disease, most notably cardiac failure and ischaemic heart disease, both directly1–3 and via its sequelae of renal failure and hypertension. Finally, the treatments for the disease, both medical and interventional, are familiar to the cardiologist, who can employ much of the knowledge and techniques used to treat coronary atherosclerosis in the management of RAS. This review describes the investigation and management of atherosclerotic renovascular disease (ARVD), with a particular focus on the aspects of most interest to the cardiologist.
The epidemiology of RAS
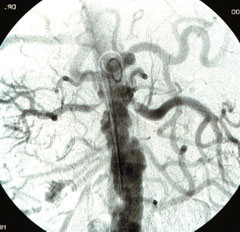
The prevalence of RAS is estimated to be around 7% in people over 65 years of age. It does not show any racial bias and its incidence increases with age.4 In patients with suspected cardiac disease requiring diagnostic coronary angiography the prevalence of renal artery stenosis is 10–15%.5-7 This is unsurprising as the degree of atherosclerosis of the renal arteries is reflective of the atherosclerotic burden in other blood vessels. ARVD tends to affect the proximal third and ostium of the renal arteries, in some cases being the extension of an aortic plaque. Figure 1 shows the typical appearance of ARVD on angiography.
This is quite unlike fibromuscular dysplasia (FMD), the next most common cause of RAS, which is a disease of the distal half of the renal artery. FMD accounts for approximately 5% of cases. It is a disease of young women, tending to occur between the ages of 15 and 50 years, and affects the distal two-thirds of the renal arteries. In contrast to ARVD, the management is uncontroversial – it is best treated with revascularisation of the affected renal artery.
The clinical characteristics of ARVD
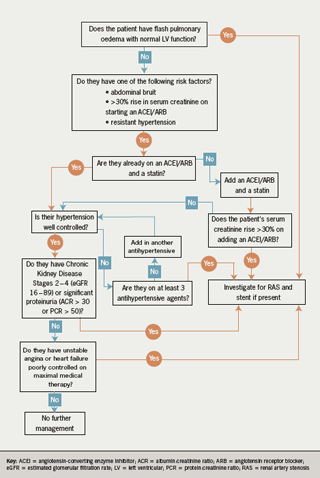
ARVD may remain asymptomatic but it may cause severe hypertension, or renal failure. Other clinical features that should prompt consideration of RAS are:
- presence of an abdominal bruit
- resistant hypertension
- a rise in serum creatinine >30% upon starting an angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor blocker (ARB)
- acute pulmonary oedema in the context of normal left ventricular (LV) function (‘flash’ pulmonary oedema).
Many of the signs of RAS could feasibly be attributed to cardiac disease or its treatment, and indeed flash pulmonary oedema is easily misdiagnosed as heart failure. Thus, it is a frequently overlooked diagnosis, especially in the context of pre-existing ischaemic heart disease or cardiac failure. Sadly, it is precisely in these patients where diagnosing ARVD is particularly important.
ARVD is associated with poorer outcomes in cardiac patients.2,8 The four-year unadjusted survival for patients undergoing coronary artery disease who have RAS with a stenosis >50% is 65% compared with 86% for those without RAS.1 Even after adjusting for the fact that patients with RAS tended to have more severe coronary artery disease and lower ejection fractions, the negative prognostic effects of RAS remain. The degree of stenosis is also important, with more severe stenosis and bilateral disease being associated with shorter survival.3 ARVD is also associated with worse LV function and increased LV hypertrophy,9 with the severity of the cardiac disease being proportional to the degree of ARVD.
The asymptomatic and non-specific presentations of ARVD have led to much difficulty in elucidating the natural history and pathophysiology of the disease. It is a progressive disease and will worsen if left untreated,10 but exactly what degree of stenosis is significant in terms of morbidity and mortality is unclear. As already discussed, more severe stenosis demonstrably leads to worse outcomes, but the same degree of stenosis will not consistently lead to the same morbidity in different patients. This is also true for coronary artery disease, but patients whose stenosis is significant usually have marker symptoms (angina, breathlessness), which is not the case with ARVD.
While ARVD may well lead to hypertension or renal dysfunction, it is important to remember that the co-existence of ARVD and hypertension or renal failure does not imply causality. This has been elegantly demonstrated by one study11 that assessed split kidney function in patients with RAS and found that in patients with unilateral RAS there was no difference between the function of the kidney with the stenosed artery and its counterpart. This suggests that the renal function is being affected by some other process, perhaps cholesterol micro-emboli, and in many cases the correlation between the severity of ARVD and the degree of renal dysfunction may be simply due to the degree of ARVD being reflective of the degree of atherosclerosis in the microvasculature.
The uncertainty about what truly constitutes significant ARVD has led to much difficulty in designing trials as different investigators have used different definitions of ‘significant stenosis’. Most choose an arbitrary measure of somewhere between 50% and 75% stenosis as significant and some will also add the criteria of hypertension resistant to treatment by two or more drugs (so-called ‘resistant’ hypertension). The result has been a great deal of confusion about the best management for ARVD.
Medical management of RAS
The management of RAS can be divided into conservative, medical, minimally invasive revascularisation and surgical. In the cardiac patient the conservative and medical strategies are essentially the same as the lifestyle recommendations are the same for both ARVD and cardiac disease. In addition, the patient is already likely to be on the first-line drugs advocated for treatment of ARVD: namely an ACE inhibitor or an ARB and a statin.12
For the purposes of this discussion we will consider ACE inhibitors and ARBs to be equivalent, an assertion supported by the recent Ongoing Telmisartan Alone and in Combination with Ramipril Global End point Trial (ONTARGET) study,13 and hereafter they are both referred to as ACE inhibitors for simplicity. As there are no data directly comparing the two mechanisms of action in patients with RAS it is impossible to be categorical about which one to use and so the use of one or the other should be dictated by physician preference, patient tolerance and local guidelines.
The role of ACE inhibitors in RAS may seem more controversial to some, as RAS was historically considered to be a contraindication to their use. In fact it is only bilateral severe RAS that is a contraindication. In patients with unilateral RAS the creatinine is likely to remain relatively stable as the unaffected kidney will provide sufficient filtration to maintain an adequate glomerular filtration rate (GFR).
Assuming the RAS is unilateral, ACE inhibitors are the rational choice of drug to treat this condition as the hypertension that ensues from RAS is due to increased renin activity. They are effective in controlling blood pressure in 70% of cases and have been shown in several studies to be more effective than other classes of antihypertensive agents.14 Furthermore, there is some evidence that they may retard the progression of renal disease15 and improve survival.16 When introducing an ACE inhibitor, especially in the context of chronic kidney disease (CKD) there may be a rise in creatinine. A rise of up to 30% is permissible and is not an indication for stopping the drug.17 Careful and frequent monitoring of electrolytes is mandatory.
If ACE inhibitors are not successful in controlling blood pressure then other classes of antihypertensive should be added. Second-line treatment, especially in patients with cardiac disease, is a beta blocker. If a beta blocker is contraindicated then calcium channel blockers should be used.15 If blood pressure is not controlled on two antihypertensives then more drugs may be added or revascularisation can be considered.
Before moving on to a discussion of revascularisation it is worth mentioning a new class of drugs, the direct renin inhibitors, of which aliskiren is the first. There are no data showing the benefit (or harm) of aliskiren in the context of RAS. However, as RAS leads to high plasma renin activity it seems reasonable to postulate that aliskiren will be particularly effective in this context. What little data there are suggest that aliskiren is non-inferior to ACE inhibitors and ARBs when used as monotherapy for hypertension control. However, when used in combination with an ACE inhibitor or an ARB it provides superior blood pressure control.18 Whether this leads to improved cardiovascular outcomes remains to be seen. As ONTARGET13 showed, lowering blood pressure further with dual blockade of the renin-angiotensin-aldosterone system does not necessarily translate into a better prognosis. At the moment it is too early to advocate routine use of aliskiren, but it seems likely that it will find a role as second- or third-line therapy in the future.
Minimally invasive revascularisation and surgical management of ARVD
Surgical management has largely been superseded by angioplasty and stenting and is only indicated in cases where stenting would be difficult or there is adjacent aorto-iliac disease that would benefit from surgery. For most patients revascularisation is done using angioplasty with or without stent placement.
However, unlike in coronary artery disease where there are widely accepted guidelines, there is no clear consensus on when percutaneous intervention is superior to medical management in ARVD except in the case of flash pulmonary oedema, a widely accepted indication for renal artery stenting.19 To date there have been only three randomised controlled trials looking at percutaneous intervention versus medical therapy.
The three trials, Essai Multicentrique Medicaments vs Angioplastie (EMMA),20 Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC)21 and one from the Scottish and Newcastle Renal Artery Stenosis Collaborative Group,22 were all fundamentally flawed: the medical regimens that patients received were sub-optimal in that many patients did not receive an ACE inhibitor and the intervention, angioplasty, was not the one that is most commonly used in clinical practice today, which is angioplasty with stent placement. None of the trials were adequately powered to detect their primary outcome measure but even so, their primary end point, control of hypertension, is not as relevant to clinicians as mortality. Even when grouped together in a meta-analysis23 no meaningful conclusions could be drawn about the effects of angioplasty on blood pressure or renal function.
Cardiologists concerned about poorer cardiac outcomes in patients with RAS will find scant evidence from these trials on whether intervention will be of any benefit: many typical cardiac patients were expressly excluded and cardiovascular outcomes were not well reported. The Scottish and Newcastle group did not detect any increase in rates of heart failure, stroke or myocardial infarction, but there were insufficient data to make any meaningful commentary.
A number of papers have reported on the benefits of renal artery stenting in patients with either cardiac failure or unstable angina but they were either case reports or retrospective series.24 In general, they suggest that renal artery stenting is beneficial and may have effects both acutely and in the medium term.25 However, there remains a dearth of hard evidence upon which to base clinical practice. Hopefully this will change when three important ongoing trials, Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL),26 Angioplasty and Stent for Renal Artery Lesions (ASTRAL)27and Stenting in Renal Dysfunction Caused by Atherosclerotic Renal Artery Stenosis (STAR),28 report their results. Preliminary results from the ASTRAL trial were presented at the Society for Cardiovascular Angiography and Interventions and the American College of Cardiology i2 summit (SCAI-ACCi2) meeting in April 2008 and these showed no differences between medical management and stenting with respect to change in kidney function, blood pressure control or the rates of major cardiovascular illness. We must wait for full data from all three trials to be published, but in the meanwhile the results from ASTRAL emphasise the need for restraint when considering revascularisation.
Despite the lack of evidence, several groups have suggested (broadly similar) guidelines on when stenting might be appropriate. For example, the American Heart Association has suggested the following as indications for revascularisation:15
- significant RAS (stenosis >50%) with malignant, accelerated or resistant hypertension or intolerance to antihypertensive medication
- significant RAS with CKD
- significant RAS with cardiac failure, unexplained pulmonary oedema or unstable angina.
Advocating stent placement in patients with CKD is contentious as the evidence to support stenting in patients with ARVD and CKD comes largely from the secondary end points of the trials described above, with additional support from retrospective observational studies.29 While renal function tends to remain stable or improve following stenting, it is worth noting that in around 30% of cases it will deteriorate.30,31 However, in general, these guidelines represent a pragmatic approach to the problems faced when treating patients with hypertension, CKD and cardiac failure, and the only other modification that one might make would be to advocate stenting in patients with bilateral RAS to enable the use of ACE inhibitors.32
Conclusion
ARVD is a condition of interest to cardiologists as it has an increased incidence in patients with coronary artery disease and is associated with a worse prognosis in patients with ischaemic heart disease and cardiac failure, independent of its effects on blood pressure and renal function. It is not clear if in all cases the degree of ARVD is a marker of other processes that confer a worse prognosis upon cardiac patients or whether ARVD directly leads to a worse outcome through an as yet undescribed mechanism.
Whatever the pathophysiology of cardiovascular damage, it is clear that patients with ARVD and cardiac disease should be treated aggressively, preferably with a regimen containing a statin and an ACE inhibitor. If the patient develops flash pulmonary oedema they should receive angioplasty and a stent.
For all other presentations of ARVD there is currently no good evidence upon which to base decisions about when revascularisation should be carried out, but it seems reasonable to try if blood pressure is poorly controlled or to permit the introduction of an ACE inhibitor, especially in patients with bilateral ARVD. Unless there is a good reason not to do so, a stent should be placed at the time of renovascular angioplasty, especially in patients with ostial lesions.
Revascularisation may also be useful in patients with CKD, advanced cardiac failure and poorly controlled angina, but in the absence of robust evidence it should only be used as a last resort. Routine screening for ARVD in cardiac patients is not advocated except in those patients where detection of ARVD is likely to result in a revascularisation procedure being carried out.
There is much to be clarified in this area but better guidelines will hopefully arrive following publication of the full results of ongoing trials, notably ASTRAL and CORAL.
Conflict of interest
None declared.
Key messages
- Renal artery stenosis is associated with an increased risk of cardiac disease and causes a worse prognosis in ischaemic heart disease and cardiac failure
- The evidence for medical treatment is good and all patients should be treated with an ACE inhibitor and a statin
- The evidence for percutaneous revascularisation is poor and it should not be used as first-line therapy
- More definitive evidence should arrive in 2008 following the publication of the CORAL, ASTRAL and STAR trials
References
- Conlon PJ, Athirakul K, Kovalik E et al. Survival in renal vascular disease. J Am Soc Nephrol 1998;9:252–6.
- Isles C, Main J, O’Connell J et al. Survival associated with renovascular disease in Glasgow and Newcastle: a collaborative study. Scott Med J 1990;35:70–3.
- Conlon PJ, Little MA, Pieper K, Mark DB. Severity of renal vascular disease predicts mortality in patients undergoing coronary angiography. Kidney Int 2001;60:1490–7.
- Kalra PA, Guo H, Kausz AT et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005;68:293–301.
- Harding MB, Smith LR, Himmelstein SI et al. Renal artery stenosis: prevalence and associated risk factors in patients undergoing routine cardiac catheterization. J Am Soc Nephrol 1992;2:1608–16.
- Weber-Mzell D, Kotanko P, Schumacher M, Klein W, Skrabal F. Coronary anatomy predicts presence or absence of renal artery stenosis. A prospective study in patients undergoing cardiac catheterization for suspected coronary artery disease. Eur Heart J 2002;23:1684–91.
- Crowley JJ, Santos RM, Peter RH et al. Progression of renal artery stenosis in patients undergoing cardiac catheterization. Am Heart J 1998;136:913–18.
- Edwards MS, Craven TE, Burke GL, Dean RH, Hansen KJ. Renovascular disease and the risk of adverse coronary events in the elderly: a prospective, population-based study. Arch Intern Med2005;165:207–13.
- Wright JR, Shurrab AE, Cooper A, Kalra PR, Foley RN, Kalra PA. Left ventricular morphology and function in patients with atherosclerotic renovascular disease. J Am Soc Nephrol2005;16:2746–53.
- Dean RH, Kieffer RW, Smith BM et al. Renovascular hypertension: anatomic and renal function changes during drug therapy. Arch Surg 1981;116:1408–15.
- Farmer CK, Cook GJ, Blake GM, Reidy J, Scoble JE. Individual kidney function in atherosclerotic nephropathy is not related to the presence of renal artery stenosis. Nephrol Dial Transplant1999;14:2880–4.
- 12. Kalra PA, Jayawardene S, Goldsmith D. Renal arterial disease. In: Goldsmith D, Ackland P, Jayawardene S (eds). ABC of kidney disease. Oxford: Blackwell-Wiley, 2007;24–27.
- Yusuf S, Teo KK, Pogue J et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547–59.
- Textor SC. ACE inhibitors in renovascular hypertension. Cardiovasc Drugs Ther 1990;4:229–35.
- Hirsch AT, Haskal ZJ, Hertzer NR et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463–e654.
- Losito A, Errico R, Santirosi P et al. Long-term follow-up of atherosclerotic renovascular disease. Beneficial effect of ACE inhibition. Nephrol Dial Transplant 2005;20:1604–09.
- Main J. Atherosclerotic renal artery stenosis, ACE inhibitors, and avoiding cardiovascular death. Heart 2005;91:548–52.
- Pasha Y, Gusbeth-Tatomir P, Covic A, Goldsmith D. Direct renin inhibitors – on target for success? Int J Urol Nephrol 2008 (in press).
- Cheung CM, Hegarty J, Kalra PA. Dilemmas in the management of renal artery stenosis. Br Med Bull 2005;73–74:35–55.
- Plouin PF, Chatellier G, Darné B, Raynaud A. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Essai Multicentrique Medicaments vs Angioplastie (EMMA) Study Group. Hypertension 1998;31:823–9.
- van Jaarsveld BC, Krijnen P, Pieterman H et al. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med 2000;342:1007–14.
- Webster J, Marshall F, Abdalla M et al. Randomised comparison of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. Scottish and Newcastle Renal Artery Stenosis Collaborative Group. J Hum Hypertens 1998;12:329–35.
- Ives NJ, Wheatley K, Stowe RL et al. Continuing uncertainty about the value of percutaneous revascularization in atherosclerotic renovascular disease: a meta-analysis of randomized trials.Nephrol Dial Transplant 2003;18:298–304.
- de Silva R, Nikitin NP, Bhandari S, Nicholson A, Clark AL, Cleland JGF. Atherosclerotic renovascular disease in chronic heart failure: should we intervene? Eur Heart J 2005;26:1596–605.
- Khosla S, White CJ, Collins TJ, Jenkins JS, Shaw D, Ramee SR. Effects of renal artery stent implantation in patients with renovascular hypertension presenting with unstable angina or congestive heart failure. Am J Cardiol 1997;80:363–6.
- Murphy TP, Cooper CJ, Dworkin LD et al. The Cardiovascular Outcomes with Renal Atherosclerotic Lesions (CORAL) study: rationale and methods. J Vasc Interv Radiol 2005;16:1295–1300.
- Mistry S, Ives N, Harding J et al. Angioplasty and STent for Renal Artery Lesions (ASTRAL trial): rationale, methods and results so far. J Hum Hypertens 2007;21:511–15.
- Bax L, Mali WPTM, Buskens E et al. The benefit of STent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by Atherosclerotic ostial stenosis of the Renal artery. The STAR-study: rationale and study design. J Nephrol 2003;16:807–12.
- Kashyap VS, Sepulveda RN, Bena JF et al. The management of renal artery atherosclerosis for renal salvage: does stenting help? J Vasc Surg 2007;45:101–08; discussion 108–09.
- Haller C. Arteriosclerotic renal artery stenosis: conservative versus interventional management. Heart 2002;88:193–7.
- Balk E, Raman G, Chung M et al. Effectiveness of management strategies for renal artery stenosis: a systematic review. Ann Intern Med 2006;145:901–12.
- Khosla S, Ahmed A, Siddiqui M et al. Safety of angiotensin-converting enzyme inhibitors in patients with bilateral renal artery stenosis following successful renal artery stent revascularization. Am J Ther 2006;13:306–08.
