Congestive heart failure (CHF) is an increasingly widespread condition, the prognosis for moderate and severe heart failure is almost identical to colorectal cancer1 and worse than breast2or prostate cancer.3 CHF has an overall population prevalence of approximately 1–3% rising to approximately 10% in the very elderly
CHF accounts for about 5% of all medical admissions and approximately 2% of total healthcare expenditure.4 Nearly one million new cases are diagnosed annually worldwide, making it the most rapidly growing cardiovascular disorder.
The consequences of heart failure for primary care are profound. CHF has been reported to be second only to hypertension as a cardiovascular reason for a surgery appointment.5Despite improvements in medical management, undertreatment is common, many patients with CHF still do not receive treatment optimised according to current guidelines.4,6
The introduction of the 2009/10 heart failure Quality Outcomes Framework (QOF) additions will bring financial incentives for the prescribing of beta blockers for patients with a diagnosis of heart failure. This will apply to all diagnosed heart failure patients. There are, however, no additional QOF points for optimising medication or maximum tolerated levels, therefore, patient care will rely on good practice and receiving treatment according to current guidelines.
The prevalence of heart failure nationally in QOF is just over 1%. Because of the increase in survival after acute myocardial infarction and ageing of the population, the number of patients with heart failure will increase rapidly in most industrialised countries. Heart failure will continue to be a challenge to healthcare.
The profile of heart failure management has been raised with the publication of the Coronary Heart Disease (CHD) National Service Framework (NSF)
Chapter 6 in 20007 and the National Institute for Health and Clinical Excellence (NICE) Heart Failure Clinical Guideline 2003.8 The heart failure publications have supported the development of community heart failure services, and heart failure specialist nurse roles.
The development of the General Practitioner with Special Interest (GPSI) in cardiology qualification and the accreditation in community echocardiography in 2004 has enabled the development of community heart failure services. The training and development of the workforce in primary care has led to improvements in the treatment and management of heart failure patients. A referral to a community specialist heart failure service or secondary care will still be relevant in certain instances, however, the 10 steps will assist in the decision to continue the management in primary care or refer for expert advice and a future management plan.
1. Make the diagnosis
Take a history to assist in determining the diagnosis of heart failure – a history of CHD (previous myocardial infarction), murmur, valve replacement, rheumatic fever, thyroid disease, atrial fibrillation and hypertension are conditions that would predispose a heart failure diagnosis. Establish the number of units of alcohol per week (consider alcoholic cardiomyopathy). The patient’s smoking history should be noted as this may suggest chronic obstructive pulmonary disease (COPD) as an alternative diagnosis and spirometry should be undertaken to rule out COPD and asthma as a cause for breathlessness.
Weigh and measure the patient, observe for possible cachexia hidden by the oedema. Enquire about shortness of breath, on exertion, at rest or at night.
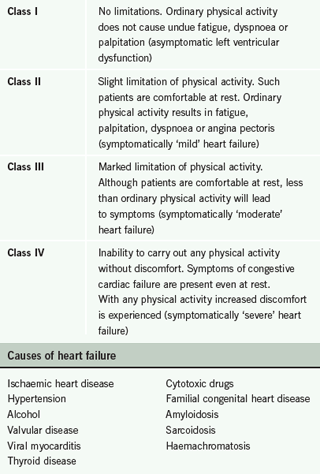
The New York Heart Association (NYHA) classification scale (table 1) can be used to classify symptom severity. Examine for any ankle, leg or abdominal oedema. Consider an alternative cause (low protein diet, renal disease, venous stasis). Examine the patient for a raised jugular venous pressure (JVP) and listen to the heart for added heart sounds or murmur. Measure the blood pressure, which may be normal or low, and take the pulse to assess whether it is irregular or fast. Could the patient be in fast atrial fibrillation which has precipitated heart failure?
Clinical assessment alone is unreliable since the symptoms and signs of heart failure may be insensitive and non-specific, however, when used in a systematic manner it can be effective in determining a diagnosis of heart failure and potentially reduce the effect on the echocardiogram service by excluding patients who are not likely to have heart failure (figure 1).9
2. Carry out the initial investigations
Take routine bloods: full blood count, urea and electrolytes (U&E), liver function tests, thyroid function and blood glucose. If brain natriuretic peptide (BNP) is available, then this will aid diagnosis (normal ranges for BNP vary with age and gender and by laboratory). The specificity of the chosen cut-off point will vary between 30% and 50%. Therefore, BNP alone is not conclusive, though it is a reliable ‘rule-out’ test. If there is a history of breathlessness (with or without oedema) indicating a heart failure diagnosis, and in addition the electrocardiogram (ECG) is abnormal, consider a trial of furosemide 40 mg daily and review in two to three days.
The patient should be reviewed with the results of the blood tests. Ensure BNP investigation is undertaken prior to commencing on furosemide, as the BNP result will be lower following diuretics. However, if the condition of the patient is unstable, then consider admission.
An ECG should be performed in every patient with suspected heart failure. Electrographic changes are common in patients suspected of having heart failure. An abnormal ECG has little predictive value for the presence of heart failure, however, if the ECG is completely normal, heart failure, especially with systolic dysfunction, is unlikely (<10%). ECG is a valuable diagnostic tool as it provides evidence of myocardial infarction or left ventricular hypertrophy (LVH).
Chest X-ray is an essential diagnostic component, indicated to exclude other causes of breathlessness. The chest X-ray is useful to detect cardiomegaly, pulmonary congestion and pleural fluid accumulation and can demonstrate the presence of pulmonary disease or infection contributing to dyspnoea. However, apart from congestion, findings are predictive only in the context of typical signs and symptoms. Cardiomegaly can be absent not only in acute but also in chronic heart failure. If ECG, chest X-ray and BNP are normal, heart failure is very unlikely. Consider other causes of breathlessness, such as overweight, unfit, smoker, anxiety, or hyperventilation.
Spirometry should be performed if there is a smoking history (smoker or passive smoker) or relevant occupational history with symptoms suggestive of COPD or asthma. If spirometry is positive, manage as per NICE COPD 2004 guidelines.10 If a diagnosis of asthma is confirmed then manage the patient according to the British Guideline on the Management of Asthma, British Thoracic Society (BTS)/Scottish Intercollegiate Guidelines Network (SIGN) guidelines.11 COPD is a frequent co-morbidity in heart failure and the prevalence ranges between 20–30%. Evaluation of natriuretic peptide levels may be helpful in this population and the negative predictive value may be most useful.
3. Arrange an echocardiogram
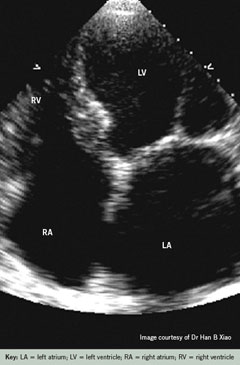
An echocardiogram is a vital diagnostic tool to support the initial suspicion of a heart failure diagnosis. Open access echocardiogram services may be available. The echocardiogram should be arranged to understand the underlying cause of the heart failure and rule out structural heart abnormalities. Only if the echocardiogram result is equivocal, the diagnosis is in doubt, the patient has a murmur and requires tertiary intervention, or the echocardiogram identifies advanced disease, possibly requiring device therapy, should the patient be referred for specialist care. If the clinician is confident to diagnose heart failure and no onward referral is required at the present time, then together with the patient and carer, a management plan will be agreed and, with the patient’s consent, medical therapy commenced – steps 4–8.
If a heart failure specialist nurse is available then consider referral to the service for a programme of patient education, self-management and support.
4. Angiotensin-converting enzyme inhibitors (ACEI)
Once a diagnosis of heart failure has been confirmed ACEI should be commenced, starting at the lowest dose once per day. The dose should be doubled at a minimum of two-week intervals to a target of the maximum tolerated dose available. The blood pressure and blood taken for U&E will be checked at seven to 14 days, prior to initiation, and following each dose increase. If the patient’s renal function is compromised, then consider stopping nephrotoxic drugs, such as non-steroidal anti-inflammatories, or if there is no congestion, reduce the loop diuretics. The patient should be monitored closely and U&E checked within one week. If the results are within acceptable limits, then continue the up-titration. However, if the renal function continues to deteriorate, then reduce the ACEI dose by half, monitor closely, checking the U&E within one week. If there is still no improvement following reduction in ACEI, stopping diuretics and nephrotoxic drugs, then consider referral to a specialist community heart failure service, or secondary care provider.
The ACEI should be stopped and a referral to a specialist service should be considered if: the potassium level is above 6.0 mmol/L or creatinine more than 350 µmol/L, or more than double the baseline reading.
5. Angiotensin II receptor antagonists (ARBs)
If the patient is intolerant of ACEI, then commence on ARBs. The evidence for ARBs is based on the Candesartan in Heart Failure – Assessment of Reduction in Mortality and Morbidity (CHARM),12 Valsartan Heart Failure Trial (VAL-Heft),13 and Valsartan in Acute Myocardial Infarction (VALIANT)14 studies. The initial dose of ARB should be the lowest available and the dose should be doubled at each step (minimum of two-weekly intervals). The dose should be halved if the patient is over 75 years or has liver impairment.
Prior to initiation and up-titration, the blood pressure and U&E should be monitored and limits applied as per ACEI instructions in step 4. The cautions apply for ACEI and ARBs, and the drugs should not be used if the patient has diagnosed renal artery stenosis, aortic/mitral valve stenosis or obstructive hypertrophic cardiomyopathy.
6. Initiate a beta blocker
Following successful initiation and up-titration to maximum dose of ACEI or ARB, then the next stage is to commence beta blockers. This should be on the condition that the patient is stable (defined by no admissions to hospital in the last month and no alteration of drug therapy in the previous two weeks). Patients who do not meet these criteria but would benefit from therapy should be referred to a specialist heart failure clinic (community clinic or secondary care). The choices of beta blocker with evidence of value in heart failure management are: bisoprolol, carvedilol, metoprolol and nebivolol. The lowest available dose should be initiated and titrated up over a period of 12 weeks. Prior to initiation of beta blocker and four days following any dose increase, the following should be observed: • the patient’s heart rate should not be lower than 50 beats per minute • the patient should not be lightheaded or dizzy • the patient’s systolic blood pressure should be more than 90 mmHg.
If any of the above applies, seek specialist advice, if not, continue to up-titrate. If the patient has a respiratory wheeze, but no weight gain, then specialist advice should be sought, or the patient referred to a community specialist clinic or secondary care heart failure service.
7. Titrating the loop diuretic dose
Increasing, and decreasing, the patient’s loop diuretic dose is key to the management of the patient’s fluid overload. The patient’s dry weight should be documented. The dry weight is defined as the patient’s stable weight with no signs of fluid overload. The patient should be weighed in similar weight clothes and at a similar time on each occasion. The loop diuretic should be up-titrated if the patient has a sudden increase in dry weight of over 1 kg which has been sustained over the previous two days, or the patient has increasing oedema and breathlessness. Furosemide should be up-titrated every three days by 40 mg at each titration. If the dry weight is still not achieved following two incremental changes, or breathlessness and oedema have not subsided, then specialist advice or referral to secondary care is required.
If the patient is prescribed bumetanide, then titrate as above, remembering that 1 mg of bumetanide is the equivalent of 40 mg furosemide. The increased dose should be maintained for three days. If the patient’s dry weight is achieved, return to the original dose. However, if there are more than two episodes of fluid overload in a two to three week period, then consider a permanent increase in diuretic dose.
The loop diuretic dose of furosemide should be decreased in 40 mg steps if the patient’s dry weight is decreased by 1 kg, sustained over two days, or urea is increased by more than 5 mmol/L, or more than 25% from baseline. The patient may complain of dizziness and thirst if dehydrated significantly. The patient’s fluid status should be assessed within 48 hours of each step change and if the patient is back to dry weight, reassess in a further 48 hours. If the patient has remained at dry weight, consider a permanent decrease in diuretic. However, if the patient is still below dry weight, then seek, specialist advice.
Specialist advice should be sought if the patient is on a regular dose of more than 160 mg furosemide.
8. Consider aldosterone antagonists
An aldosterone antagonist can be considered if the patient remains symptomatic – the patient describes symptoms that classify as NYHA II–IV, despite treatment with an ACEI, a diuretic and, where indicated, a beta blocker (based on randomized aldosterone evaluation study [RALES] trial).15
U&E should be checked prior to initiation, and also one week after initial dose. Before commencing on spironolactone 25 mg once per day, potassium supplements and potassium-sparing diuretics should be discontinued.
However, if creatinine is more than 200 µmol/L, or urea is more than 11–12 mmol/L and/or potassium is more than 5.5 mmol/L, then seek specialist advice/refer.
One week after initiation, if the creatinine is less than 200 µmol/L, urea less than 18 mmol/L and potassium less than 5.5 mmol/L, in addition the patient has no diarrhoea or vomiting, then continue 25 mg spironolactone and monitor U&E every four weeks for three months, then every three months for six months and every six months thereafter. The target dose of spironolactone is 25–50 mg once daily. If the patient experiences significant gynaecomastia, then eplerenone at the same dose can be used as a replacement, clinical monitoring and treatment regimen remains the same.
If the creatinine level is more than 200 µmol/L, urea more than 18 mmol/L and potassium is more than 5.9 mmol/L, then consider reducing to 12.5 mg daily, re-check U&E in two weeks, however, if concerned regarding the result, then seek advice/refer.
9. Palliative care
The prognosis of patients with advanced heart failure is often very difficult to determine, as there is no simple method for measuring organ function accurately in clinical practice. There are many factors causing exacerbation of heart failure, however, these can be improved if the correct therapies are instituted in time. In addition, a proportion of heart failure patients will die suddenly (sudden cardiac death [SCD]). This is generally unpredictable, although the majority of heart failure patients will die from progressive cardiac pump dysfunction. These uncertainties about prognosis need to be explained and discussed openly with the patient, their families and carers, as even those confidently identified as ‘end-stage’ may include some who recover and improve and others who die prematurely through SCD. Thus, true ‘end-stage’ is reached when: the patient is chronically and severely symptomatic (NHYA III or IV), and no further conventional therapy is available that will provide any realistic prospect of improvement without incurring undue risks to the patient.16
Therefore, what needs to be considered is whether such therapies will enhance the quality of life of an individual patient. If this is in doubt, such cases should be referred to a specialist service for further investigation and clarification.
10. Heart failure nurse role
The previous nine steps outline the diagnosis and management of heart failure, and when to seek specialist advice or refer patients for specialist management. As outlined, careful monitoring is vital and intensive. This role is ideally undertaken jointly with GPs and specialist heart failure nurses in the community where this role is available. The heart failure nurse can offer the patient and carers ongoing support, education and self-management, e.g. daily weight measurement, and reduced salt and fluid intake. The heart failure nurse specialist will discuss advice on lifestyle, smoking cessation, alcohol consumption, diet and physical activity, including sexual activity and the sexual positions less likely to strain a patient with a heart failure diagnosis.
The heart failure nurse can discuss the diagnosis and prognosis, palliative and end-of-life care, following discussion with the GP, patient, carer and family.
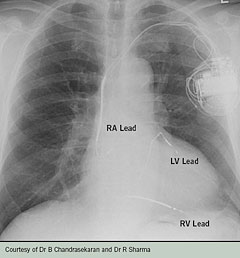
In areas where heart failure rehabilitation is available, the heart failure nurse can assess the patient’s condition and together with the patient and rehabilitation/physiotherapist, devise a heart failure rehabilitation programme to improve the patient’s functional capacity. The implantation of a cardiac resynchronisation device or an implantable defibrillator (figure 2) can be life-saving and improve the quality of the patient’s life. The heart failure nurse specialist can assess against NICE criteria for device therapy17,18 and discuss referral to a specialist cardiologist with the GP, patient and carer. The patient can be assessed for surgical options, e.g. revascularisation and/or heart transplantation. This assessment will be undertaken by a specialist cardiologist.
Conflict of interest
None declared.
References
- Joint Report of the West Midlands, Director of Public Health and the West Midlands Regional Cancer Registry. Cancer and health. Birmingham: WMRHA, 1995:210.
- Joint Report of the West Midlands, Director of Public Health and the West Midlands Regional Cancer Registry. Cancer and health. Birmingham: WMRHA, 1995:71.
- Joint Report of the West Midlands Director of Public Health and the West Midlands Regional Cancer Registry. Cancer and health. Birmingham: WMRHA, 1995:82.
- McMurray JJW, Stewart S. The burden of heart failure. Eur Heart J 2002;4(Suppl D):D50–D58.
- O’Connell JB, Bristow MR. Economic impact of heart failure in the United States: time for a different approach. J Heart Lung Transplant 1994;13:S107–S112.
- McMurray JJ. Failure to practice evidence-based medicine: why do physicians not treat patients with heart failure with angiotensin-converting enzyme inhibitors? Eur Heart J 1998;19(Suppl L):15–21.
- Department of Health. Coronary heart disease national service framework. London: DoH, 2000.
- National Institute of Health and Clinical Excellence. Clinical Guideline 5. Management of chronic heart failure in adults in primary and secondary care. London: NICE, July 2003.
- Chambers J, Fuat A, Liddiard S et al. Community echocardiography for heart failure. Br J Cardiol 2004;11:399–402.
- National Institute of Health and Clinical Excellence. Clinical Guideline 12. Management of chronic obstructive pulmonary disease in adults in primary and secondary care. London: NICE, 2004.
- British Thoracic Society (BTS), Scottish Intercollegiate Guideline Network (SIGN). British guideline on the management of asthma: a national clinical guideline. Thorax 2008;63(Suppl IV):Iv1–Iv121.
- Pfeffer MA, Swedberg K, Granger CB et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet 2003;362:759–66.
- Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667–75.
- Pfeffer MA, McMurray JJV, Velazquez EJ et al.; for the Valsartan in Acute Myocardial Infarction Trial Investigators. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003;349:1893–906.
- Pitt B, Zannad F, Remme WJ et al.The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709–17.
- West Yorkshire Cardiac Network 2008. Symptom management guidelines for patients in the later stages of heart failure and criteria for referral to specialist palliative care. Available from: http://www.yorksandhumberhearts.nhs.uk/upload/WYCN/Guidelines/Symptom%20Management%20Guidelines%20%20HF%20Jul%2008%20Version%202.pdf
- National Institute of Health and Clinical Excellence. Technology appraisal 120 guidance. Heart failure cardiac resynchronisation. London: NICE, 2007.
- National Institute of Health and Clinical Excellence. Technology appraisal 95 guidance. Arrhythmia: implantable cardioverter defibrillators (review of TA11 guidance). London: NICE, 2008.
