Introduction
Stable angina is the most common manifestation of coronary heart disease. While considered relatively benign in terms of prognosis, the condition confers a higher risk of cardiovascular events than in the general population, with average annual mortality rates of 1–2%.
Guidelines for the management of stable angina are relatively conservative in their approach, given their process of development. Moreover, stable angina management has not been as rigorously evaluated in large randomised trials as other coronary conditions. The role of newer treatment options in management algorithms also merits wider consideration. This expert round table ‘Chronic stable angina guidelines – is there an emerging international consensus?’ was held at the Royal Society, London, on 27th January 2012. It was convened by the British Journal of Cardiology to discuss these issues, with the aim of achieving consensus on the most appropriate management of patients with stable angina within Europe. This supplement summarises the round table meeting discussions.
Round table meeting panel
Chairs
Professor Jose Lopez-Sendon
Hospital Universitario La Paz, Idi Paz, Madrid, Spain
Dr Henry Purcell
Editor, BJC/Royal Brompton Hospital, UK
Panel
Professor Paolo Camici
Università Vita-Salute San Raffaele, Milan, Italy
Dr Caroline Daly
St James Hospital, Dublin, Ireland
Professor Jamil Mayet
International Centre for Circulatory Health,
St Mary’s Hospital, Imperial College London, UK
Dr John Parissis
University of Athens, Greece
Professor Francesco Pelliccia
University of Roma La Sapenza, Rome, Italy
Professor Christophe Piot
Arnaud de Villeneuve University Hospital, Montpellier, France
External reviewer
Professor Rainer Hambrecht
Heart Centre of Bremen, Germany
The scope of the problem in 2012
Stable angina, assessed according to the Rose Angina Questionnaire, is estimated to affect about 6% of individuals (both men and women) across 31 countries.1 The condition, already common in industrialised societies, such as Western Europe and the USA, is predicted to further increase in the future. In part, this is due to an increasingly ageing society given that the likelihood of ischaemic heart disease increases with age.2 Improved management of acute coronary syndromes (ACS) has also contributed to increased longevity of patients with chronic ischaemic disease. The economic implications of these predictions are substantial, despite the growing need for financial restraint in healthcare systems.
The Euro-Heart Survey provides insights into how the management of stable angina has evolved. The survey involved 3,779 community-based patients (mean age 61 years, 58% male) recruited by 197 centres across Europe at first presentation to the cardiologist during 2002–2003. Initial treatment mainly comprised aspirin and a beta blocker, with about 50% of patients receiving a statin. After assessment, more than 50% of patients were on two or more anti-anginal treatments. On follow-up, 12% of patients were managed conservatively, 13% underwent revascularisation within four weeks and the remainder either awaited clinical review or underwent further testing.3 Exercise testing was the most consistently used assessment, although functional imaging was reported in 15–30% of centres, depending on the availability of facilities on-site. Despite this, clinical symptoms and signs were the strongest predictors for prognosis.4
 Moving forward to 2011–2012, there appears to have been little change in the prevalence of stable angina, although cardiovascular mortality rates have decreased. The American College of Cardiology National Cardiovascular Data Registry shows slightly more than one third of nearly 400,000 patients with suspected coronary disease who underwent elective cardiac catheterisation had obstructive coronary artery disease. Therefore, better strategies for risk stratification are needed to inform decisions and to increase the diagnostic yield of cardiac catheterisation in routine clinical practice.5
Moving forward to 2011–2012, there appears to have been little change in the prevalence of stable angina, although cardiovascular mortality rates have decreased. The American College of Cardiology National Cardiovascular Data Registry shows slightly more than one third of nearly 400,000 patients with suspected coronary disease who underwent elective cardiac catheterisation had obstructive coronary artery disease. Therefore, better strategies for risk stratification are needed to inform decisions and to increase the diagnostic yield of cardiac catheterisation in routine clinical practice.5
Undoubtedly the availability of rapid access chest pain clinics has improved identification of patients with angina at high coronary risk. Despite this, about one-third of patients were still misdiagnosed, more likely those who were younger or of South Asian descent, or had atypical symptoms or a normal resting ECG, highlighting a clear need for improvement.6 There is also some reluctance to refer patients for revascularisation.7 Similarly, there is well-documented, considerable underdiagnosis of angina in women compared to men.1 As a result, the burden of angina remains substantial, and confers increased disability, and correspondingly reduced quality of life.7,8
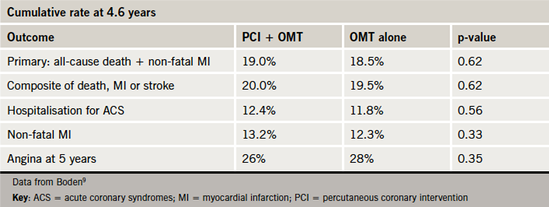
In terms of management options, the COURAGE (Clinical Outcomes Utilising Revascularisation and Aggressive Drug Evaluation) trial findings fuelled debate regarding the value of revascularisation.9 The overall COURAGE results suggested no difference in clinical outcomes in stable angina patients allocated an initial strategy of percutaneous coronary intervention (PCI) on top of optimal medical therapy versus optimal medical therapy alone. Additionally, just over one-quarter of patients in each arm had persistence of angina at follow-up (table 1). However, the rate of additional revascularisation procedures was significantly lower in patients who underwent revascularisation on top of optimal medical therapy (21.1% vs. 32.6%, p<0.001). Several caveats need to be considered: most patients in the revascularisation arm of COURAGE underwent only partial or incomplete revascularisation; 32% of patients in the medical arm subsequently crossed-over to PCI; and there was an excess of male patients (~85%) in the study.
Data from COURAGE-PCI10 showed significantly greater reduction in myocardial ischaemia (where ischaemia is defined as substantial changes in ST-segment depression or T-wave inversion on the resting electrocardiogram or inducible ischaemia with either exercise or pharmacologic vasodilator stress) in patients allocated to PCI versus medical therapy alone (33.3% vs. 19.8% in the optimal medical therapy arm had ≥5% reduction in myocardial ischaemia, p=0.004). Indeed, the extent of myocardial ischaemia was a key determinant of the benefit (both in terms of reduction in ischaemia and symptoms) obtained from revascularisation. Subsequent observational data reaffirmed that the clinical benefit derived from early revascularisation is confined to patients with significant ischaemia (>15%) (figure 1). In contrast, medical therapy without early revascularisation was associated with superior clinical benefits in patients with minimal ischaemia.11,12
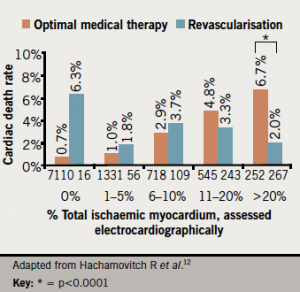
A large retrospective analysis of more than 10,000 patients referred for myocardial perfusion scintigraphy suggested that the benefits of revascularisation were confined to those patients with inducible ischaemia of 10% or more of the left ventricular myocardium.13
These findings clearly impact the use of functional imaging in patient management. Increased and earlier use of functional imaging has improved proficiency in identifying patients most likely to benefit from revascularisation. Routine measurement of fractional flow reserve during angioplasty has also been shown to improve clinical outcomes compared with traditional angiography-guided treatment.14 Most recently, SPARC (Study of Myocardial Perfusion and Coronary Anatomy Roles in CAD), an observational registry study, showed that the use of cardiac catheterisation and medical therapy increased in proportion to the extent of abnormal imaging findings in patients at intermediate to high risk of coronary artery disease (CAD). Despite this, it is clear that many patients at greatest risk receive less than optimal treatment. In SPARC, up to 68% were not referred for catheterisation, up to 30% did not receive aspirin, slightly less than one-half did not receive a beta blocker, and 20% to 25% were not receiving a lipid-lowering agent 90 days after testing.15

COURAGE has not appeared to change practice. Evidence of non-optimised treatment of patients with stable CAD undergoing PCI was shown in one observational study from the US. This showed that less than half of the patients were receiving optimal medical therapy before PCI and approximately two-thirds were receiving optimal medical therapy at discharge following PCI. This confirmed that there was relatively little change in practice patterns after publication of the COURAGE trial.16
Thus, while the management of stable angina in Europe has certainly improved during the last decade, a number of challenges remain (table 2). There is clearly a need for new approaches to improve patient risk stratification and management.
Current guidelines: need for consensus
Guidelines aim to provide evidence-based recommendations to help clinicians in the routine management of patients. However, their remit also encompasses the requirements of regulatory agencies, academic societies, patients, payers and healthcare providers, as well as cost issues.
For stable angina, the process is made more difficult by the limitations of the data. Many of the trials of medical therapy versus interventional approaches that supported guideline recommendations were carried out almost three decades ago, when routine secondary prevention approaches used differed vastly from those in practice today. Furthermore, there have been major advances in interventional approaches influencing the need for repeat treatment.
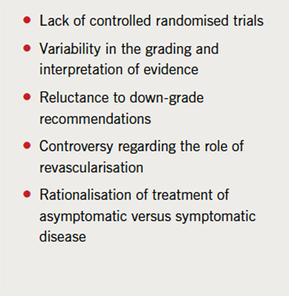
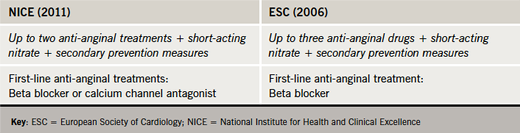
Different approaches are taken in reviewing the evidence on which guideline recommendations are based. For example, the European Society of Cardiology (ESC) assesses evidence ranging from A (i.e. evidence derived from multiple randomised controlled trials or meta-analyses) to C (consensus of expert opinion, small studies and/or registry or retrospective data). In contrast, NICE (National Institute for Health and Clinical Excellence) uses the GRADE approach, also taking into account the quality of evidence, methodological limitations, dose-response relationship and the consistency of effect across studies. Thus, even with the same evidence base, guideline groups may differ in their interpretation of the evidence and hence level of recommendations. This may explain why a proportion of the ESC recommendations for stable angina were supported by level A evidence, whereas none of those in the US guidelines were graded level A, although based on the same evidence.17,18 This also highlights difficulties in achieving agreement in practice across different countries, an important limitation to international consensus in the management of stable angina (see table 3).
Evolving guidelines: NICE 2011
 Guidelines need to evolve to reflect recent progress. Given that the ESC guidelines were published in 2006, review is clearly indicated and most likely imminent. The availability of newer agents, such as ranolazine or ivabradine, increased understanding of the importance of secondary prevention measures, and advances in interventional techniques all provide impetus for change.
Guidelines need to evolve to reflect recent progress. Given that the ESC guidelines were published in 2006, review is clearly indicated and most likely imminent. The availability of newer agents, such as ranolazine or ivabradine, increased understanding of the importance of secondary prevention measures, and advances in interventional techniques all provide impetus for change.
NICE recently published new recommendations for the management of stable angina.19 These new guidelines follow on from recent revision of guidelines for diagnosis of chest pain of recent onset.20 The key priorities recognised by NICE were provision and support for individuals with stable angina; medical therapy; and the role of investigation and revascularisation.
Diagnosis of chest pain
As defined by NICE,20 clinical assessment plus diagnostic testing are needed to confirm ischaemia and establish diagnosis. The likelihood of CAD is the starting point to indicate the need for other or more invasive tests. For individuals in whom the likelihood of CAD is <10%, other causes of chest pain should be considered. If stable angina cannot be excluded or diagnosed, a 12-lead resting ECG is useful for indicating changes consistent with CAD or prior ischaemia or infarction. Stratification for the likelihood of CAD is crucial for determining the need for diagnostic functional imaging (table 4). If there are features characteristic of typical angina, and the estimated likelihood of CAD is >90%, the individual should be treated as for stable angina without the need for further diagnostic tests.
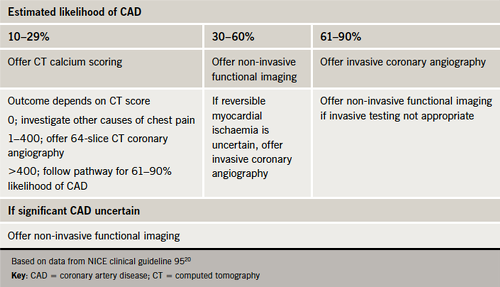
However, there are several issues with this pathway. It is implicit that among lower risk patients, the likelihood of CAD may be overestimated. Not only does this have obvious cost implications, but there also may be concerns regarding cumulative radiation doses in the light of recent data.21 Clearly, there is a need to balance such concerns within the clinical context, of particular relevance in lower-risk middle-aged women. NICE does not recommend the use of exercise ECG for diagnosis of stable angina in individuals without known CAD. Therefore, in clinical practice, the role of exercise ECG is increasingly relegated to assessing functional capacity in patients with established CAD. Early risk stratification is crucial to identifying patients who would benefit from more intensive treatment or an interventional approach.
Management
The medical management pathway recommended by NICE is summarised in figure 2. These recommendations focus on the relative merits of medical therapy and revascularisation, and the role each strategy has in improving quality of life, morbidity and mortality. Symptom control is a key objective.
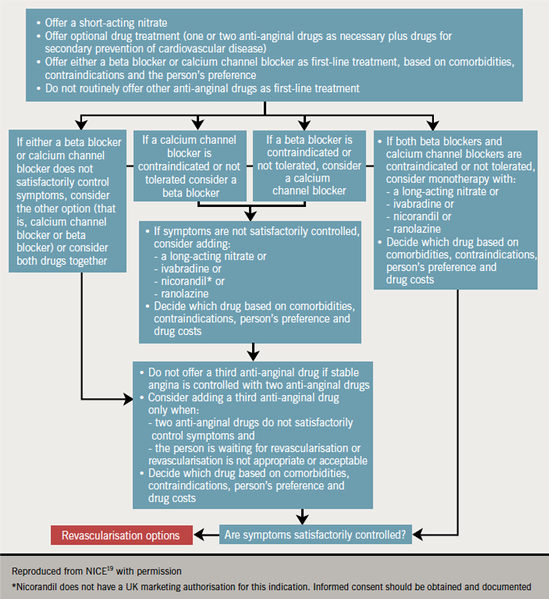
Optimal medical therapy should comprise one or two anti-anginal agents as necessary. In contrast to the ESC guidelines (2006),17 which do not support the use of calcium channel blockers other than rate-lowering calcium channel blockers as an alternative to beta blockers in the post-MI setting, the NICE guidelines do not differentiate between the use of beta blockers or calcium channel blockers as first-line anti-anginal therapy (table 5). If initial treatment is ineffective or poorly tolerated, the guidelines recommend switching between these treatments; combination therapy may be indicated if symptoms are not satisfactorily controlled. Adding a third anti-anginal drug should be avoided in controlled patients and instead reserved for situations where patients remain inadequately controlled on dual therapy, or are awaiting revascularisation.
The guidelines also emphasise the importance of secondary prevention measures, including lifestyle modification (increased activity, stopping smoking and adoption of a Mediterranean-style diet). However, routine secondary prevention therapy is not indicated for individuals with suspected cardiac syndrome X.
Further investigation and revascularisation are indicated if the patient remains inadequately controlled on optimal medical therapy. The choice of revascularisation procedure is dependent on the complexity of underlying coronary artery disease (with or without the involvement of left main stem), as well as evidence of other cardiovascular risk factors (age or diabetes). Invasive procedures may be also of value in individuals satisfactorily controlled by optimal medical therapy but with a high likelihood of prognostically significant CAD, including left main stem or proximal three vessel disease, taking into account the risks and benefits of the investigation.
Key management priorities in the NICE guidelines include provision of information and support for individuals with stable angina. The guidelines stress that patients should be treated consistently irrespective of age, sex or ethnicity.
NICE 2011: a significant therapeutic advance over ESC 2006?
The NICE guidelines provide a pragmatic view by placing beta blocker and calcium channel blocker treatments on an equal footing. If anything, the NICE guidelines are more enthusiastic about the use of calcium channel blockers as the initial therapy, despite the most recent supportive evidence derived from INVEST (International Verapamil SR-Trandolapril Study) in 2003.22 Additionally, most of the evidence reviewed mainly related to older agents in each class. In contrast, the ESC guidelines tend to favour the use of a beta blocker as an initial therapy. The rationale for this approach is supported by consideration of the underlying pathophysiology of stable angina, as well as clinical evidence to support the use of beta blocker therapy as an anti-arrhythmic agent, in heart failure and to reduce sudden death. There is also preliminary evidence to suggest benefit in coronary microvascular dysfunction underlying regional ischaemia.23,24 Further, the ESC believe that the evidence does not show that calcium channel blockers are disease-modifying, as their use does not change the risk of MI, sudden cardiac death, or all-cause mortality. Evidence from ACTION (A Coronary Disease Trial Investigating Outcome with Nifedipine GITS), the largest study to date in stable angina (n=7,665) does not support the use of nifedipine added to beta blocker therapy in patients with concomitant hypertension.25
In both guidelines, the long-acting nitrates are recommended as a second-line option, largely on an empirical basis. Common side effects include headache, light-headedness, flushing, palpitations and hypotension. Combination with phosphodiesterase type 5 inhibitors should be avoided. However, there are a number of concerns with the use of these agents. These include the development of tolerance to their haemodynamic and anti-anginal effects during sustained therapy. and possibly, cross-tolerance to sublingual nitrates. In addition, trials have highlighted potential adverse effects in post-MI patients.26 Consequently, these agents are recommended only for symptom relief as a short-term therapy or as complementary therapy to beta blockers and/or calcium channel blockers.17,22
Evolving role for newer anti-anginal treatments
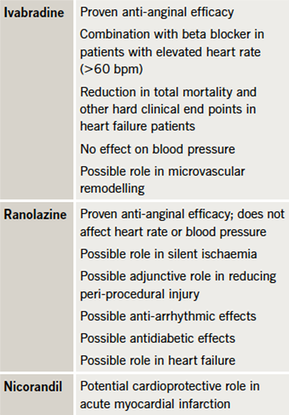
While both the ESC and NICE guidelines indicate a second-line role for the newer anti-anginal treatments in the management pathway, new data suggest a need for re-evaluation (table 6). Indeed, there is some evidence to suggest the possibility that at least some of these agents may also act as ‘disease modifiers’.
Ivabradine
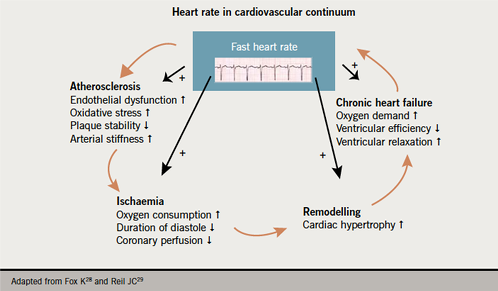
Ivabradine acts on the If current which regulates the intrinsic pacemaker activity of the sinoatrial node, resulting in reduction in heart rate and myocardial oxygen demand. Ivabradine also offers benefits beyond those associated with beta blocker therapy, with an effect on coronary dilation resulting in greater increase in coronary blood flow and hence maximisation of oxygen supply, and preservation of myocardial contractility and relaxation resulting in better ventricular filling and cardiac adaptation. Anti-anginal efficacy has been proven in clinical studies.27 As high resting heart rate (HR) is a cardiovascular risk factor (figure 3), and associated with increased myocardial ischaemia, it would be appropriate to target HR reduction using ivabradine. Such an approach has been associated with improved clinical outcomes.28,29
Additionally, in heart failure patients, HR reduction with ivabradine has been shown to improve clinical outcomes, including reduction in death from heart failure in the SHIFT (Systolic Heart Failure Treatment with the IfInhibitor Ivabradine Trial).30 Experimental studies also suggest a possible role in microvascular remodelling.
The European Medicines Agency (EMA) have approved ivabradine as second-line therapy for patients unable to tolerate or with a contraindication to beta blockers or in combination with beta blockers in patients inadequately controlled with an optimal dose and whose HR is > 60 beats per minute (bpm); as well as to improve outcomes in chronic heart failure in patients with resting HR ≥75 bpm.31,32
Ranolazine
Ranolazine inhibits the late sodium influx across the sarcolemma, thereby attenuating abnormalities in ventricular repolarisation and contractility associated with myocardial ischaemia, as well as preventing calcium overload that causes cardiac ischaemia. Thus, ranolazine interrupts the positive feedback loop that perpetuates myocardial ischaemia and myocardial dysfunction. Ranolazine does not significantly alter heart rate or systolic blood pressure; for this reason, ranolazine could be of particular value in individuals with angina who are unable to increase other anti-anginal medications due to their haemodynamic effects.
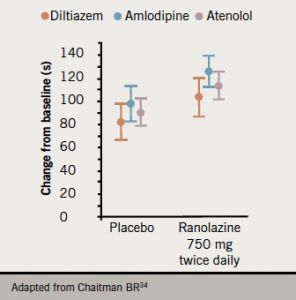
The anti-anginal efficacy of ranolazine has been demonstrated in clinical trials (figure 4).33,34 In Europe, ranolazine is currently indicated as add-on therapy for the symptomatic treatment of patients with stable angina pectoris who are inadequately controlled or intolerant to first-line anti-anginal therapies (such as beta blockers and/or calcium antagonists), while in the US it has also been approved as first-line therapy.35
New findings suggest additional therapeutic applications. It has been proposed that ranolazine may have a role in silent ischaemia,36 as well as an adjunctive role in reducing myocardial injury during PCI.37 Given that more than 25% of patients who undergo PCI will develop microemboli during the procedure, this suggests a potential additional therapeutic role for ranolazine. Based on experimental studies, this may be mediated via vasodilatory effects (without systemic hypotension) and/or an effect on alpha-1 receptors. Additionally, data from the MERLIN-TIMI 36 (Metabolic Efficiency with Ranolazine for Less Ischemia in non-ST Elevation Acute Coronary Syndromes) trial showed that treatment with ranolazine reduced the risk of ischaemia, and there was also indication of anti-arrhythmic effects, in particular significantly reducing the incidence of episodes of ventricular tachycardia lasting ≥8 beats.38 Ranolazine has similar benefits in diabetic and non-diabetic patients and, in addition, it has been shown to significantly improve glycaemic control in patients with diabetes.39,40 Ranolazine has also been investigated for a possible role in heart failure based on findings from the MERLIN-TIMI 36 trial,41 and an exploratory study, RALI-DHF.42,43
For the above reasons, ranolazine could be useful in angina patients complicated by other comorbidities such as diabetes, arrhythmia and heart failure with preserved ejection fraction.
Nicorandil
Nicorandil is a potassium channel activator, as well as acting as a nitrate-like epicardial coronary vasodilator, lowering preload through venodilation. Treatment is associated with improved myocardial function during ischaemia-reperfusion, protection of the myocardium during ischaemia, shortened action potential duration, and prevention of intracellular calcium toxicity, of relevance in modulating ischaemic cell damage. Nicorandil can be combined safely with nitrates, calcium antagonists or beta blockers but additive anti-anginal effects have yet to be demonstrated.
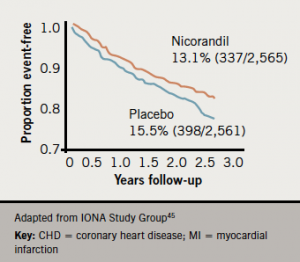
Experimental evidence suggests that activation of mitochondrial potassium channels may be a critical target for cardioprotection against myocardial reperfusion injury.44 Clinical studies lend support to this concept. In the IONA (Impact Of Nicorandil in Angina) study in 5,126 patients with angina, treatment with nicorandil significantly improved clinical outcomes (figure 5).45 Supportive data from the OACIS registry showed reduction in post-event mortality.46 However, in the J-WIND-KATP study,47 nicorandil had no effect on creatine kinase release or left ventricular ejection fraction in patients undergoing reperfusion treatment for acute MI. Among a number of limitations, the dose used may have been too low to show an effect. Further data may help to delineate a role for nicorandil beyond angina management.
Improving guidance – is future consensus possible?

It is clear that there is not yet international consensus for the management of stable angina. A number of outstanding issues need to be addressed to achieve this (table 7).
Co-morbidities
Most patients with stable angina will be older and likely to have multiple co-morbidities. Data from CLARIFY (Prospective Observational Longitudinal Registry of Patients with Stable Coronary Artery Disease) show that hypertension, dyslipidaemia, heart failure, diabetes and peripheral vascular disease are common in these patients (figure 6).48 This impacts treatment options, and also contributes to compliance issues. Indeed, there is recent evidence that older patients with stable angina – although representing a substantial proportion of the burden of disease – are not benefitting from improvement in management to the same extent as seen in younger patients.49 Such patients may be less likely to receive optimal medical therapy given existing contraindications and potential interactions with other treatments.
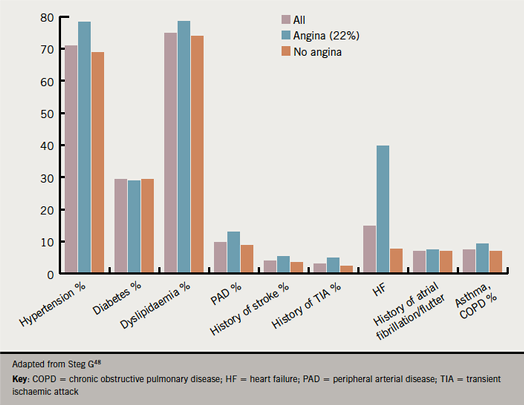
The treatment limitations imposed by such co-morbidities are summarised in table 8. As shown by CLARIFY, almost one-third of patients with stable angina will have diabetes. In these patients, treatments other than a beta blocker may be preferred. Indeed, of the newer agents, ranolazine may be considered, given evidence of comparable efficacy in diabetic and non-diabetic patients, as well as the lack of detrimental effects on glycaemic control.40
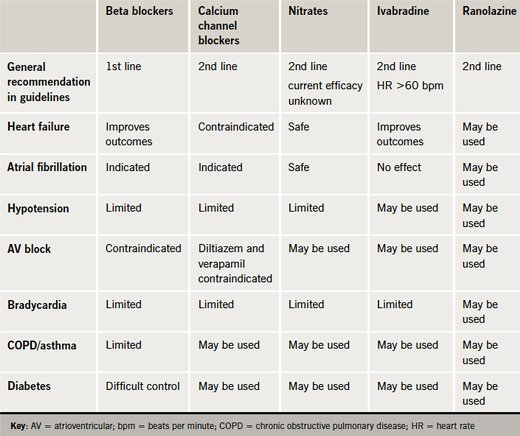
First-line therapy
First-line treatment should not only provide symptomatic relief, but also address the underlying disease mechanism(s). There is clear support for the use of beta blockers as first-line therapy, given evidence of disease-modifying effects and benefits as an anti-arrhythmic agent, in heart failure and to reduce sudden death. In contrast, there is a lack of evidence that calcium channel blockers are disease-modifying. Consequently, it is likely that the use of calcium channel blockers will be down-graded in new ESC recommendations.
Second-line options
In previous guidelines, the long-acting nitrates have been recommended as a second-line option, based on their pharmacological profile as a vasodilator. However, given that randomised controlled trials are still lacking, this therapeutic approach remains largely empirical. The mechanisms underlying the vasodilatory action of these agents are also not fully understood. In clinical practice, the propensity for development of toleranceis a limitation for use;26 indeed, evidence from clinical trials implicates tolerance in adverse outcomes in chronic CAD patients.50 Consequently the use of the long-acting nitrates should be down-graded in future guidelines.Newer anti-anginal treatments which exhibit a neutral haemodynamic profile and potential cardioprotective properties can bridge the gap between nitrate use and effective management of stable angina and ischaemia.
Ranolazine, ivabradine or nicorandil are already indicated as second-line options in patients inadequately controlled by first-line treatment or in whom such treatment is contraindicated or not tolerated. Consideration of patient co-morbidities suggests that ranolazine and ivabradine will have increasing roles in the management of older patients with stable angina (see table 8). In addition, ivabradine in combination with a beta blocker is indicated for the management of patients inadequately controlled with an optimal dose of beta blocker and whose HR is >60 bpm. As reviewed above, data also suggest further potential; for ranolazine, as an antiarrhythmic agent or for preventing myocardial injury during PCI, and for ivabradine, for a potential role in heart failure, based on findings from SHIFT (Systolic Heart Failure Treatment with Iƒ Inhibitor Ivabradine Trial).
Secondary prevention
Both the ESC and NICE guidelines emphasise the importance of secondary prevention treatments, in addition to first-line anti-anginal therapy. Despite this, implementation is often less than optimal. For example, in the recent FORGET (Failure to Recognise the Therapy in Angina Pectoris) study, a significant proportion of patients with chronic stable angina did not achieve therapeutic targets for lipid and blood pressure control.51 This is likely to be especially problematic in the older patient with multiple co-morbidities. Whether targeting other lipid targets may provide further benefit in patients with stable CAD is contentious, awaiting the results of ongoing major prospective trials.
Coronary revascularisation
Finally, there is a need for further discussion about the role of coronary revascularisation in individuals who remain symptomatic despite optimal medical therapy, and who do not have evidence of high-risk features (left main or multi-vessel disease). It is important to emphasise that revascularisation should be seen as complementary to, rather than instead of, optimal medical therapy.
Further insights will undoubtedly come from the ongoing ISCHEMIA trial (www.ischemiatrial.org). This is a large randomised multi-centre, multi-national trial, randomising patients with extensive myocardial ischaemia between optimal medical therapy and PCI. This ‘mega trial’ will hopefully answer the question of which strategy is superior in the treatment of patients with symptomatic CAD.
Conclusion
Stable ischaemic heart disease is a major public health challenge. Despite the recent introduction of new UK guidelines in 2011, there remain a number of unresolved issues, in particular, disparities in the management of the older patient with multiple co-morbidities.
It is anticipated that new ESC guidelines will reaffirm the value of beta blocker therapy as first-line therapy, and by consequence down-grade the role of the calcium channel blockers. Additionally, it is likely that given the patient profile, there will be an increasing role for the newer anti-anginal treatments, ranolazine and ivabradine, in clinical pathways for the management of stable angina.
References
- Hemingway H, Langenberg C, Damant J, Frost C, Pyörälä K, Barrett-Connor E. Prevalence of angina in women versus men: a systematic review and meta-analysis of international variations across 31 countries. Circulation 2008;117:1526–36. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.720953
- World Health Organization. The Atlas of Heart Disease and Stroke. The future of CVD. Available at: http://www.who.int/cardiovascular_diseases/en/cvd_atlas_25_future.pdf . [Accessed 29 February 2012].
- Daly CA, Clemens F, Sendon JL, et al. The initial management of stable angina in Europe, from the Euro Heart Survey: a description of pharmacological management and revascularization strategies initiated within the first month of presentation to a cardiologist in the Euro Heart Survey of Stable Angina. Eur Heart J 2005;26:1011–22. http://dx.doi.org/10.1093/eurheartj/ehi109
- Daly CA, De Stavola B, Sendon JL, et al. Predicting prognosis in stable angina – results from the Euro Heart Survey of stable angina: prospective observational study. BMJ 2006;332:262–7. http://dx.doi.org/10.1136/bmj.38695.605440.AE
- Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010;362:886–95. http://dx.doi.org/10.1056/NEJMoa0907272
- Sekhri N, Feder GS, Junghans C, Hemingway H, Timmis AD. How effective are rapid access chest pain clinics? Prognosis of incident angina and non-cardiac chest pain in 8762 consecutive patients. Heart 2007;93:458–63. http://dx.doi.org/10.1136/hrt.2006.090894
- Buckley B, Murphy AW. Do patients with angina alone have a more benign prognosis than patients with a history of acute myocardial infarction, revascularisation or both? Findings from a community cohort study. Heart 2009;95:461–7. http://dx.doi.org/10.1136/hrt.2008.146944
- Hemingway H, Vahtera J, Virtanen M, Pentti J, Kivimäki M. Outcome of stable angina in a working population: the burden of sickness absence. Eur J Cardiovasc Prev Rehabil 2007;14:373–9. http://dx.doi.org/10.1097/01.hjr.0000230106.01396.a2
- Boden WE, O’Rourke RA, Teo KK, et al. COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–16. http://dx.doi.org/10.1056/NEJMoa070829
- Shaw LJ, Berman DS, Maron DJ et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008;117:1283–91. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.743963
- Hachamovitch R, Rozanski A, Shaw LJ, et al. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J 2011;32:1012–24. http://dx.doi.org/10.1093/eurheartj/ehq500
- Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation 2003;107:2900–07. http://dx.doi.org/10.1161/01.CIR.0000072790.23090.41
- Pfisterer ME, Zellweger MJ, Gersh BJ. Management of stable coronary artery disease. Lancet 2010;375:763–72. http://dx.doi.org/10.1016/S0140-6736(10)60168-7
- Tonino PAL, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary angiography. N Engl J Med 2009;360:213–24. http://dx.doi.org/10.1056/NEJMoa0807611
- Hachamovitch R, Nutter B, Hlatky MA, et al. Patient management after noninvasive cardiac Imaging results from SPARC (Study of Myocardial Perfusion and Coronary Anatomy Imaging Roles in Coronary Artery Disease). J Am Coll Cardiol 2012;59:462–74. http://dx.doi.org/10.1016/j.jacc.2011.09.066
- Borden WB, Redberg RF, Mushlin AI, Dai D, Kaltenbach LA, Spertus JA. Patterns and intensity of medical therapy in patients undergoing percutaneous coronary intervention. JAMA 2011;305:1882–9. http://dx.doi.org/10.1001/jama.2011.601
- Fox K, Alonso Garcia MA, Ardissino D, et al. Guidelines on the management of stable angina pectoris: full text. The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J 2006. http://dx.doi.org/10.1093/eurheartj/ehl002
- Fraker TD Jr, Fihn SD; 2002 Chronic Stable Angina Writing Committee; American College of Cardiology; American Heart Association, Gibbons RJ et al. 2007 chronic angina focused update of the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 guidelines for the management of patients with chronic stable angina. J Am Coll Cardiol 2007;50:2264–74. http://dx.doi.org/10.1016/j.jacc.2007.08.002
- National Institute for Health and Clinical Excellence. NICE clinical guideline 126. Management of stable angina. July 2011. Available at http://www.nice.org.uk/CG126 [accessed 10 February 2012].
- National Institute for Health and Clinical Excellence. NICE clinical guideline 95. Chest pain of recent onset: Assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. March 2010. Available at: http://guidance.nice.org.uk/CG95 [accessed 15 February 2012].
- Einstein AJ, Weiner SD, Bernheim A, et al. Multiple testing, cumulative radiation dose, and clinical indications in patients undergoing myocardial perfusion imaging. JAMA 2010;304:2137–44. http://dx.doi.org/10.1001/jama.2010.1664
- Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003;290:2805–16. http://dx.doi.org/10.1001/jama.290.21.2805
- Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology and management. Circulation 2010;121:2317–25. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.900191
- Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007;356:830–40. http://dx.doi.org/10.1056/NEJMra061889
- Poole-Wilson PA, Lubsen J, Kirwan BA et al; Coronary disease Trial Investigating Outcome with Nifedipine gastrointestinal therapeutic system investigators. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment (ACTION trial): randomised controlled trial. Lancet 2004;364:849–57. http://dx.doi.org/10.1016/S0140-6736(04)16980-8
- Heidenreich PA, McDonald KM, Hastie T, et al. Meta-analysis of trials comparing ß-blockers, calcium antagonists, and nitrates for stable angina. JAMA 1999;281:1927–36. http://dx.doi.org/10.1001/jama.281.20.1927
- Tardif JC, Ford I, Tendera M, Bourassa MG, Fox K; INITIATIVE Investigators. Efficacy of ivabradine, a new selective I(f) inhibitor, compared with atenolol in patients with chronic stable angina. Eur Heart J 2005;26:2529–36. http://dx.doi.org/10.1093/eurheartj/ehi586
- Fox K, Ford I, Steg PG, Tendera M, Ferrari R; on behalf of the Beautiful Investigators. Ivabradine for patients with stable coronary artery disease and left ventricular dysfunction (BEAUTIFUL): a randomized, double-blind, placebo-controlled trial. Lancet 2008;372:807–16. http://dx.doi.org/10.1016/S0140-6736(08)61170-8
- Reil JC, Bohm M. BEAUTIIFUL results – the slower, the better? Lancet 2008;372:779-80. http://dx.doi.org/10.1016/S0140-6736(08)611172-1
- Swedberg K, Komajda M, Böhm M et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010;376:875–85. http://dx.doi.org/10.1016/S0140-6736(10)61198-1
- NeLM news service. European CHMP supports license extension for ivabradine (Procoralan®). 28 September 2009. Available at: http://www.nelm.nhs.uk/en/NeLM-Area/News/2009—September/28/European-CHMP-supports-license-extension-for-ivabradine-Procoralan/?query=IVABRADINE&rank=44. [Accessed 29 February 2012].
- NeLM news service. European CHMP recommends approval of license extension for ivabradine (Procoralan) as treatment of chronic heart failure (NYHA II to IV) and updates contra-indications. 16 December 2011. Available at: http://www.nelm.nhs.uk/en/NeLM-Area/News/2011—December/16/European-CHMP-recommends-approval-of-license-extension-for-ivabradine-Procoralan-as-treatment-of-chronic-heart-failure-NYHA-II-to-IV-and-updates-contra-indications/?query=IVABRADINE&rank=35 . [Accessed 29 February 2012].
- Chaitman BR, Skettino SL, Parker JO, et al; MARISA Investigators. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol 2004;43:1375–82. http://dx.doi.org/10.1016/j.jacc.2003.11.045
- Chaitman BR, Pepine CJ, Parker JO, et al; for the CARISA Investigators. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA 2004;291:309–16. http://dx.doi.org/10.1001/jama.291.3.309
- NeLM news service. European CHMP adopts positive opinion on ranolazine (Latixa) for the treatment of stable angina pectoris. 25 April 2008. Available at: http://www.nelm.nhs.uk/en/NeLM-Area/News/494080/494359/494371/?query=RANOLAZINE&rank=33 . [Accessed 29 February 2012].
- Conti CR. Ranolazine and silent ischemia. J Am Coll Cardiol 2011;58:1083. [letter] http://dx.doi.org/10.1016/j.jacc.2010.11.084
- Pelliccia F, Marazzi G, Pasceri V, et al. Usefulness of preprocedural therapy with ranolazine to prevent myocardial damage in percutaneous coronary intervention. Abstract: P2355, European Society of Cardiology Congress, Aug/Sept 2011. Available at: http://spo.escardio.org/AbstractDetails.aspx?id=98382&eevtid=48. [Accessed 29 February 2012].
- Scirica BM, Morrow DA, Hod H, et al. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation 2007;116:1647–52. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.724880
- Morrow DA, Scirica BM, Chaitman BR, et al. Evaluation of the glycometabolic effects of ranolazine in patients with and without diabetes mellitus in the MERLIN-TIMI 36 randomized controlled trial. Circulation 2009;119:2032–9. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.763912 .
- Timmis AD, Bernard R, Chaitman BR, et al. Effects of ranolazine on exercise tolerance and HbA1c in patients with chronic angina and diabetes. Eur Heart J. 2006;27:42–8. http://dx.doi.org/10.1093/eurheartj/ehi495
- Morrow DA, Scirica BM, Sabatine MS, et al. B-type natriuretic peptide and the effect of ranolazine in patients with non–ST-segment elevation acute coronary syndromes. Observations from the MERLIN–TIMI 36 trial. J Am Coll Cardiol, 2010;55:1189–96. http://dx.doi.org/10.1016/j.jacc.2009.09.068
- Jacobshagen C, Belardinelli L, Hasenfuss G, Maier LS. Ranolazine for the treatment of heart failure with preserved ejection fraction: background, aims, and design of the RALI-DHF study. Clin Cardiol. 2011;34:426–32. http://dx.doi.org/10.1002/clc.20897
- Maier L, Wachter R, Edelmann F, et al. Ranolazine for the treatment of diastolic heart failure in patients with preserved ejection fraction: results from the RALI-DHF study. J Am Coll Cardiol 2012;59:865. http://dx.doi.org/10.1016/S0735-1097(12)60866-3
- Tsuchida A, Miura T, Tanno M et al. Infarct size limitation by nicorandil: roles of mitochondrial K(ATP) channels, sarcolemmal K(ATP) channels, and protein kinase C. J Am Coll Cardiol 2002;40:1523–30. http://dx.doi.org/10.1016/S0735-1097(02)02268-4
- IONA Study Group. Effect of nicorandil on coronary events in patients with stable angina: the Impact Of Nicorandil in Angina (IONA) randomised trial. Lancet 2002;359:1269–75. http://dx.doi.org/10.1016/S0140-6736(02)08265-X
- Sakata Y, Nakatani D, Shimizu M, et al. Oral treatment with nicorandil at discharge is associated with reduced mortality after acute myocardial infarction. J Cardiol 2012;59:14–21. http://dx.doi.org/10.1016/j.jjcc.2011.08.001
- Kitakaze M, Asakura M, Kim J, et al. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomized trials. Lancet 2007;370:1483–93. http://dx.doi.org/10.1016/S0140-6736(07)61634-1
- Steg PG. Baseline data of the CLARIFY registry. Presented at the European Society of Cardiology Congress, Paris, August–September, 2011.
- Macchia A, Romero M, D’Ettorre A, Mariani J, Tognoni G. Temporal trends of the gaps in post-myocardial infarction secondary prevention strategies of co-morbid and elderly populations vs. younger counterparts: an analysis of three successive cohorts between 2003 and 2008. Eur Heart J 2012;33:515–22. http://dx.doi.org/10.1093/eurheartj/ehr410
- Nakamura Y, Moss AJ, Brown MW, Kinoshita M, Kawai C. Long-term nitrate use may be deleterious in ischemic heart disease: A study using the databases from two large-scale postinfarction studies. Multicenter Myocardial Ischemia Research Group. Am Heart J 1999;138:577–85. http://dx.doi.org/10.1016/S0002-8703(99)70163-8
- Elder DH, Pauriah M, Lang CC, et al. Is there a Failure to Optimize theRapy in anGina pEcToris (FORGET) study? QJM 2010;103:305–10. http://dx.doi.org/10.1093/qjmed/hcq011

