This year’s 7th Annual Scientific Meeting of the Cardiorenal Forum looked at diabetes and other co-morbidities in relation to cardiorenal disease. The meeting was held at the Royal Institute of British Architects, London, October 5th 2012, and endorsed by the Renal Association and the British Society for Heart Failure. Michael Pope and Hugh McIntyre report on its highlights.
Introduction
As doctors and scientists we are accustomed to breaking down problems and simplifying complex pathology in order to focus our management and identify possible targets for future therapies. The pathophysiology of cardiorenal disease is no different but, as yet, attempts to elucidate the complex interaction between heart and kidneys has failed.
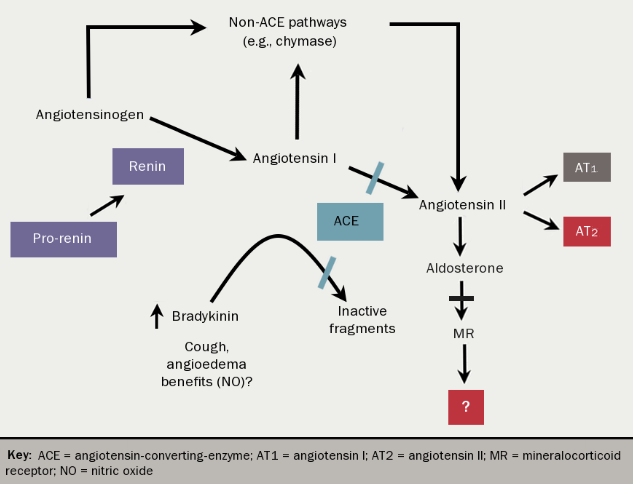
Although cardiac and renal disease are often diagnosed together, it is clear that a straightforward causal relationship does not exist. Disease in either serves as a risk factor for disease in the other and perpetuates the progression of that disease, but why this is so is unclear. Whilst the renin-angiotensin-aldosterone system (RAAS) (see figure 1) certainly plays its part and therapeutic strategies targeted at inhibiting this system have been successful, much of the pathophysiology remains unexplained.
The Cardiorenal Forum was created in response to a realisation that success in the management of cardiorenal disease would only come about by a change of perspective that explored cardiological and nephrological pathologies together. The forum’s 7th Annual Scientific meeting focussed on diabetes and other comorbidities, exploring how these may provide targets for future therapeutic interventions.
Inflammation
There is increasing evidence that inflammation plays an important role in the pathophysiology of both renal and cardiac disease.1 Dr Paul Audhya (Reata Pharmaceuticals, Irving, Texas, USA) described the inflammatory cascade as a “deterministic chaos”, meaning that there is no one pathway with multiple steps making up the inflammatory process, but rather a multitude of highly complex pathways, none of which are distinct from each other. These pathways interact in numerous ways, and with effects which can be impossible to identify, or ascribe to one clear molecular or chemical process. The process cannot easily be broken down into its constituent parts, or manipulated with pharmacological interventions.
Numerous markers of inflammation have been identified as playing a part in cardiac and renal disease, including tumour necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), human soluble TNF-receptor type 1 (sTNF-R1), and C-reactive protein (CRP). Inflammation is clearly an early component of endothelial activation, involved in atheromatous plaque formation, as well as both renal and myocardial fibrosis and remodelling resulting in heart failure and reduced glomerular filtration. However, attempts at targeting these inflammatory pathways with drug therapies have so far been unsuccessful.
Randomised controlled trials of TNF blockers in heart failure were stopped early due to fears of worsening outcomes. Beyond their well-recognised effect on low-density lipoprotein (LDL) cholesterol, statins have been shown to decrease oxidative stress and subsequently decrease TNF-α, IL-1β, interleukin 6 (IL-6), soluble vascular-cell adhesion molecule-1 (sVCAM-1) and von Willebrand factor (vWF) resulting in a reduction of number of inflammatory cells and stabilisation of atheromatous plaques. Despite this clearly anti-inflammatory effect, they have no benefit in heart failure.
A novel anti-oxidant derivative of oleanolic acid, bardoxolone, which exerts its anti-inflammatory effects via inhibition of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) was shown to significantly increase estimated glomerular filtration rate (GFR), however, in patients with moderate-to-severe chronic kidney disease (CKD) and type 2 diabetes mellitus in the BEAM study (Bardoxolone Methyl Treatment: Renal Function in CKD/Type 2 Diabetes).2 Unfortunately the phase 3 BEACON trial of bardoxolone methyl in patients with stage 4 CKD and type 2 diabetes has recently been terminated early, due to excess adverse events and mortality in the treatment arm.
Diabetes
The microvascular and macrovascular complications of diabetes are well known, as are their effects on renal and cardiovascular disease. However, although at first sight this relationship appears clear – hyperglycaemia resulting in endothelial damage, inflammation, fibrosis, and subsequent renal dysfunction and atheromatous plaque formation – the truth is far more chaotic. The microvascular complications are considered specific to diabetes, but are not an inevitable consequence of hyperglycaemia. In type 1 diabetes, retinopathy is largely related to glycaemic control, but nephropathy is not.
It is observed that patients either suffer nephropathy or they don’t, and whilst a genetic cause for this has been postulated, no responsible genes have been identified. Similarly, type 2 diabetes is increasingly recognised as not simply a disease of glucose regulation but rather a systemic disease of metabolism. Macrovascular complications are not specific to diabetes, but type 2 diabetes is an independent risk factor and is the most common cause of end stage renal disease in the UK.
Professor Steve Bain (Swansea NHS Trust and University of Wales) drew attention to the “golden years cohort”. This group of patients with type 1 diabetes, treated with insulin for 50–70 years, had an average glycated haemoglobin (Hba1c) of 7.6% and yet are observed to suffer no complications of their diabetes. Other characteristics of this group are that they are generally lean, require low insulin doses, and have elevated high-density lipoproteins (HDL), in stark contrast to the characteristics of the metabolic syndrome of which type 2 diabetes is a part.
Remarkably, 35.7% of these patients have micro or macroalbuminuria. Micro- and subsequently macroalbuminuria are considered the first detectable signs of renal dysfunction in diabetes and predate the deterioration in GFR. Screening for these is therefore considered important to allow instigation of therapies such as angiotensin-converting-enzyme (ACE) inhibitors and angiotensin 2 receptor antagonists in order to delay the decrease in GFR.
However, the observation of an abnormal albumin/creatinine ratio in the golden years cohort, despite a low risk of significant renal dysfunction and the fact that patients often have hypertension and microalbuminuria at the time of diagnosis of diabetes, may suggest a further pathological entity in addition to CKD.3 Furthermore, the risk to patients with diabetes, hypertension and albuminuria is really of cardiac disease/death, rather than renal disease itself. Should the goal of therapy therefore be preventing cardiac death in such patients, not just delaying progression of renal disease? In this context, does screening for microalbuminuria in patients with diabetes, hypertension and known cardiac disease really provide extra information to guide management?
Aldosterone
As already highlighted, the RAAS (figure 1) is really the only significant pathophysiological pathway in cardiorenal disease that we have been able to successfully manipulate for therapeutic gains. However, it is becoming increasingly clear that we do not fully understand the effect of upregulation of this system and the pathophysiological effect of increased circulating aldosterone is clearly far beyond that of fluid and electrolyte balance. Similarly, we’ve come to appreciate that aldosterone production is not purely an effect of circulating angiotensin II. Indeed, ACE inhibitors are known to cause an initial drop in both angiotensin II and aldosterone, but this rebounds with a slow increase in aldosterone.4
This may in part be the result of aldosterone synthase induction in the renal cortex, which as well as occurring in response to angiotensin II, is also potentiated by a low sodium intake, high blood sugars and possibly high blood lipids. Equally, we know that aldosterone has both paracrine and autocrine effects beyond its well-recognised endocrine actions when produced by endothelial cells, vascular smooth muscle, blood pressure control areas of the brain, and the renal cortex. Beyond the well-studied endocrine actions, which remain unclear, there is emerging evidence of an association between aldosterone and mineral bone disorders.
Recent efforts to elucidate the true pathological effects of aldosterone increasingly focus on inflammation and fibrosis. We know from animal studies that an infusion of aldosterone will promote an inflammatory response with increased circulating pro-inflammatory cytokines, formation of reactive oxygen species (the harbingers of oxidative stress), and growth factors such as the ubiquitous transforming growth factor beta (TGF-β). This results in fibroblast proliferation and activation, increased collagen production, and fibrosis.5
That this has such significant repercussions for cardiac and renal disease is not surprising. On the vasculature, this causes arteriosclerosis and stiffening, exposing the myocardium to grossly abnormal loading conditions leading to left ventricular hypertrophy. On the heart, it causes fibrotic remodelling thereby adversely affecting myocardial contractility. On the kidneys, it causes glomerulosclerosis and renal scarring with progressive loss of nephrons and fall in GFR. As this reduction in GFR progresses, the normal relationship between high extracellular volume and low aldosterone becomes distorted and in patients with end stage kidney disease on dialysis, levels of circulating aldosterone are grossly elevated. This, perhaps, goes some way to explaining the accelerated vascular and cardiac pathology observed in this group of patients and why the leading causes of death in end stage renal disease are heart failure and sudden cardiac death.
Dr John Townend (University Hospital Birmingham NHS Foundation Trust) pointed out that the heart, although obviously a muscle, could also be viewed as a “collagenous skeleton in which myocytes exist”, with an approximately 3:1 ratio of fibroblasts to myocytes and complete turnover of cardiac collagen every three to four weeks. Interruption of this dynamic process could explain the strong benefits of mineralocorticoid receptor (MR) antagonists in heart failure demonstrated by both the RALES (Randomised Aldactone Evaluation Study) and EMPHASIS-HF (Eplerenone in Mild Patients Hospitalisation and Survival Study in Heart Failure) studies. Indeed, further studies have shown spironolactone to decrease left ventricular mass index, as well as reduce arterial stiffness and increase aortic distensibility, even in patients who were not hypertensive and had a normal left ventricular mass at baseline.
The fact that mineralocorticoid receptors are found in the preglomerular vasculature, mesangial cells, fibroblasts and adipocytes – key areas in regulating fibrosis – again highlights the significance of aldosterone in CKD. Studies in animal models have shown that aldosterone blockade reduces renal scarring, as well as myocardial and aortic fibrosis.6 It was thus postulated that dual blockade of the RAAS may provide further benefit to renal function in diabetic nephropathy. A recent study showed no benefit of lisonopril and irbesartan, however, although the study was grossly underpowered.7 In recognising the importance of aldosterone for driving progression of kidney disease through renal remodelling, should we be rethinking our approach to spironolactone in this group of patients? Since we know that ACE inhibitors slow the progression of CKD, might MR antagonists be shown to have this effect too, and would it necessitate a reappraisal of our approach to hyperkalaemia?
Heart failure with a preserved ejection fraction (HF-PEF) is another pathological entity where the involvement of the RAAS is less clear and traditional therapies demonstrate much less efficacy. The novel angiotensin neprilysin inhibitor LCZ696 was recently shown to produce greater reduction of the N-terminal prohormone of brain natriuretic peptide (NT-proBNP), a marker of left ventricular stress, when compared with the angiotensin receptor antagonist valsartan in patients with HF-PEF.8 Further studies will be required to examine whether this translates into improved clinical endpoints. In addition a study investigating the effect of spironolactone in patients with HF-PEF is due to report next year.
The bulk of current evidence points to aldosterone as being involved in much of the ‘chaos’ that is central to cardiorenal disease. In the context of the high salt intake in modern western diets, it was proposed that aldosterone has in many ways now become a pathological rather than physiological hormone. Should we even be using aldosterone antagonists in place of ACE inhibitors? Ethics may preclude studies to that effect, but should we at least consider MR antagonists in place of angiotensin receptor blockers in patients who are unable to tolerate ACE inhibitors?
Iron, magnesium, and vitamin D
The metabolism of iron, magnesium and vitamin D may be collateral damage of the chaos of cardiorenal disease. Anaemia is a well-recognised element of CKD that has adverse effects on cardiac prognosis and has been discussed at previous meetings of this forum. The role of iron alone, however, is less clear. The FAIR-HF (Ferinject Assessment in Patients with Iron Deficiency and Chronic Heart Failure) study showed a functional improvement when iron supplementation was given to patients with heart failure even without a rise in haemoglobin concentrations. Iron deficiency can be difficult to define and diagnose but can be present in both heart failure and CKD with or without anaemia. Whether replacing iron in this context will genuinely be beneficial is yet to be fully elucidated.
Low levels of magnesium and vitamin D have been implicated in numerous disease processes. Women with a high magnesium intake have been suggested to have a decreased risk of the metabolic syndrome, and a high intake may decrease the risk of diabetes and even lower blood glucose in patients with diagnosed type 2 diabetes. Similarly, low serum magnesium levels have been associated with higher all-cause and cardiovascular mortality. Much of the benefit seems potentially due to its effect on blood pressure, but an association with atherosclerosis and of course arrhythmia have also been examined.
Supplementation has been shown to reduce blood pressure and enhance the effect of antihypertensive drugs. One study looked at the effect of magnesium on coronary artery disease and stroke, which showed a beneficial effect on stroke only in patients who were hypertensive. Again, a potential interaction with inflammation has been suggested, possibly through inhibition of cholesterol oxidation and vessel calcification. The role in calcification is most intriguing in end stage renal disease patients in whom high serum magnesium has been shown to suppress parathyroid hormone (PTH) excretion. The difficulty is once again in translating these experimental and epidemiological observations into clinical practice.
Similarly, vitamin D deficiency (widely reported to be associated with cardiovascular disease in recent years) has been implicated in inflammation, and low vitamin D levels are associated with increased arterial sclerosis and less compliant vessels. Very low vitamin D levels are well recognised in end stage renal disease. Again, however, a recent study on the effect of paracalcitol in patients with a GFR of 15–60 ml per minute per 1.73 m2 (not on dialysis) on left ventricular mass index and echocardiographic parameters of diastolic dysfunction over 48 weeks, failed to showed benefit.9
Conclusion
Much of the discussion in this forum highlighted how our traditional methods of identifying steps in a pathophysiological pathway and attempting to manipulate them has failed to yield the desired effects in the field of cardiorenal medicine. Is this a sign that our traditional methods cannot be applied here? The pathology is not one of a simply distorted physiological pathway but of a pathological chaotic distortion of an already chaotic physiological state. No pathway works in unison and no pathology affects a universal pathway.
Having said this, however, the overarching theme of most of the day’s talks was inflammation. In recognising that the only real success story in cardiorenal management has been our manipulation of the RAAS, we are focussing more on aldosterone and are realising its importance in inflammation. If we are to make further progress, we need to evaluate fully the role of this “physiological-turned-pathological” hormone and develop new strategies to antagonise its effects.
Diary date
The 8th Annual Scientific Meeting of the Cardiorenal Forum has been provisionally scheduled for 4th October 2013. Further details will be available at
www.cardiorenalforum.com
Michael Pope
FY2 Doctor
Queen Alexandra Hospital, Portsmouth, PO6 3LY
Hugh McIntyre
Consultant Cardiologist
Conquest Hospital, Hastings, East Sussex TN37 7RD
Correspondence to:
([email protected])
References
1. Cachofeiro V, Goicochea M, de Vinuesa SG, Oubina P, Lahera V, Luno J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl 2008;111:S4–S9. http://dx.doi.org/10.1038/ki.2008.516
2. Pergola PE, Raskin P, Toto RD, et al. for the BEAM Study Investigators. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 2011;365:327–36. http://dx.doi.org/10.1056/NEJMoa1105351
3. Bain SC, Gill GV, Dyer PH, et al. Characteristics of type 1 diabetes of over 50 years duration (the Golden Years Cohort). Diabetic Medicine 2003;20:808–11. http://dx.doi.org/10.1046/j.1464-5491.2003.01029.x
4. Staessen J, Lijnen P, Fagard R, Verschueren LJ, Amery A. Rise in plasma concentration of aldosterone during long term angiotensin II suppression. J Endocrinol 1981;91:457–65. http://dx.doi.org/10.1677/joe.0.0910457
5. Brown NJ. Aldosterone and vascular inflammation. Hypertension 2008;51:161–7. http://dx.doi.org/10.1161/HYPERTENSIONAHA.107.095489
6. Rocha R, Stier CT Jr, Kifor I et al. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology 2000;141:3871–8. http://dx.doi.org/10.1210/en.141.10.3871
7. Fernandez Juarez G, Luno J, Barrio V, et al; PRONEDI study group. Effect of dual blockade of the renin-angiotensin system on the progression of type 2 diabetic nephropathy: a randomized trial. Am J Kidney Dis 2012. http://dx.doi.org/10.1053/j.ajkd.2012.07.011
8. Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012;380:1387–95. http://dx.doi.org/10.1016/S0140-6736(12)61227-6
9. Thadhani R, Appelbaum E, Pritchett Y, et al. Vitamin D therapy and cardiac structure and function in patients with chrinic kidney disease: the PRIMO randomised controlled trial. JAMA 2012;307:674–84. http://dx.doi.org/10.1001/jama.2012.120
