Typical echocardiographic assessment of left ventricle (LV) size is based on single-dimensional measurements at mitral valve leaflet tips. In ischaemic and non-ischaemic cardiomyopathy (ICM and NICM) and aortic regurgitation (AR) where spherical remodelling is observed, this single-dimensional measurement at the LV base may underestimate LV volume. We hypothesised the maximum diameter would provide a closer approximation. A retrospective analysis of 1,680 consecutive cardiovascular magnetic resonance (CMR) examinations identified 82 patients with substantial LV dilation (LVEDVi >130 ml/m2) and 23 controls. LV end-diastolic and end-systolic diameters were measured using echocardiography and CMR at the standard level (EDDMV and ESDMV) and the maximum diameter (EDDmax and ESDmax). Indexed diameters were fitted to indexed volumes using cubic regressions. Maximum diameters had higher R2 values in fitting LV volume, and improved categorisation of subjects with chamber enlargement without substantially increasing the false-positive rate. Standard measurements may underestimate LV volume in cases of spherical remodelling, use of the maximum dimension may be a straightforward approach to improve assessment of LV volume and remodelling.
Introduction
In volume overload states, such as aortic regurgitation (AR), ischaemic cardiomyopathy (ICM), and non-ischaemic cardiomyopathy (NICM), spherical dilation occurs.1 Khouri et al. described four remodelling patterns based on the indexed left ventricle (LV) end-diastolic volume and LV concentricity.2
Standard LV chamber quantification, according to the American Society of Echocardiography (ASE) guidelines, involves obtaining a single-dimensional measurement of the LV diameter from the parasternal long-axis acoustic window at the level of mitral valve leaflet tips. Although it is the combination of qualitative and quantitative measurements that guides the final clinical management, this measurement has several management implications.3,4 We hypothesised that in cases of spherical dilation, this measurement may underestimate the extent of remodelling compared with the measurement at the widest level, due to altered geometry.
Materials and methods
We retrospectively analysed 1,680 patients who underwent cardiovascular magnetic resonance (CMR) and echocardiograms at our institution between January 2007 and April 2010. The study was Institutional Review Board approved, with waiver of individual informed consent. The time interval between CMR and echocardiography was within a month in 74% of patients (median interval: one day). CMR reports were reviewed for subjects with marked LV dilation (indexed end-diastolic volume [EDV] ≥130 ml/m2) in the absence of significant atrioventricular valve insufficiency on echocardiography. Subjects where the exam indication was AR and with regurgitant fraction >10% (mean=32%) on CMR were classified as AR. Subjects where the exam indication was a viability study with qualitatively mild-moderate regurgitation or less on CMR were classified as cardiomyopathy. They were subcategorised into ICD or NICD based on ischaemic damage on delayed gadolinium-enhanced imaging. Control subjects were obtained by selecting patients from the same institutional records as above during the same time period that had normal indexed EDV on CMR (63–98 ml/m2 for males, 57–92 ml/m2 for females),5 an ejection fraction ≥55%, <5% regurgitant fraction by CMR, and no evidence of ischaemic damage. Further, these patients had normal blood pressure and no significant abnormal findings on other cardiovascular testing (e.g. catheterisation, nuclear imaging).
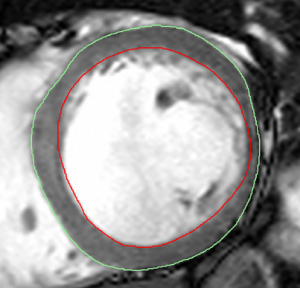
(CMR). Volumes and masses were computed using the steady-state free precession short-axis stack by drawing endocardial (red) and epicardial (green) contours
All patients underwent CMR on 1.5 T magnetic resonance scanners. After initial scout images were obtained, turbo spin echo and gradient echo imaging was performed for anatomic definition. Dynamic cine imaging (steady-state free precession) was performed to analyse cardiac chamber anatomy, wall-motion, and qualitative valvular function, and velocity-encoded phase contrast flow quantification sequences were obtained to assess haemodynamics. Viability studies included 0.2 mmol/kg of gadolinium-chelate contrast intravenously (Magnevist) to assess ischaemic damage. Transthoracic echocardiography was performed by experienced sonographers using standardised protocols.
EDV, end-systolic volume (ESV), and myocardial mass were measured on the steady-state free precession short-axis stack by drawing epicardial and endocardial contours excluding papillary muscles (figure 1). Aortic flow and regurgitant fraction was computed using phase-contrast flow quantification. Using the long-axis parasternal view, end-diastolic diameter and end-systolic diameter were measured on echocardiographic images at the level of the mitral valve tips (EDDMV and ESVMV) using 2D calliper,3 and at the level perceived to yield the largest diameter (EDDmax and ESVmax). These diameters were also measured using CMR using an equivalent (three-chamber) view.
The EDDmax/EDDMV and ESDmax/ESDMV ratios were computed for both CMR and echocardiography. The EDDmax/EDDMV and ESDmax/ESDMV ratios were compared between groups using the Kruskal-Wallis test. Among subjects with remodelling, the correlation between the percentage increase of EDDmax from EDDMV measured on echocardiography and indexed LV volumes measured on magnetic resonance imaging (MRI) was calculated.
Diameters measured at mitral valve tips and at the maximum diameter during systole and diastole on echocardiography were indexed by the subject’s height. End-diastolic and systolic volumes were indexed using body surface area (BSA).2,6,7 Indexed diameters were fitted to indexed volumes by cubic polynomial regression and R2 values were computed. Absolute and indexed end-diastolic diameters were used to predict the presence of LV dilation (LVEDV/BSA >98 ml/m2 for males, >92 ml/m2 for females).
Statistical analyses were performed using JMP 9.0 software (SAS Institute, Cary, NC) and R version 3.0.1 (GNU Public License). A significance level of 0.05 was used for all statistical tests.
Results
There were 82 subjects that fit criteria for AR, ICM, and NICM, and 23 control subjects were included in the analysis. Mean age was 53.2 ± 16.8 years. Subjects with ICM and NICM had lower ejection fraction (EF) than controls or subjects with AR. Patients in the AR group were younger and had significantly higher regurgitant fraction than the other groups (table 1). LVEDV was much larger in subjects with spherical remodelling compared with controls. LVESV was smaller in subjects with AR compared to ICM and NICM.

The difference between maximum LV diameter and the diameter measured at mitral valve tips was greater in subjects with LV dilation compared with controls (table 2). Comparing the ratio of EDDmax/EDDMV across all groups using the Kruskal-Wallis test, a significant difference was observed on echocardiography (p=0.0466) and CMR (p=0.0024). For ESDmax/ESDMV, a significant difference was present for CMR (p=0.001), but not for echocardiography (p=0.17). Among patients with LV remodelling, EDDmax was 9.8% greater than EDDMV on average on echocardiography. The correlation coefficient between this percentage difference and indexed LV volume was 0.134 with no apparent relationship.

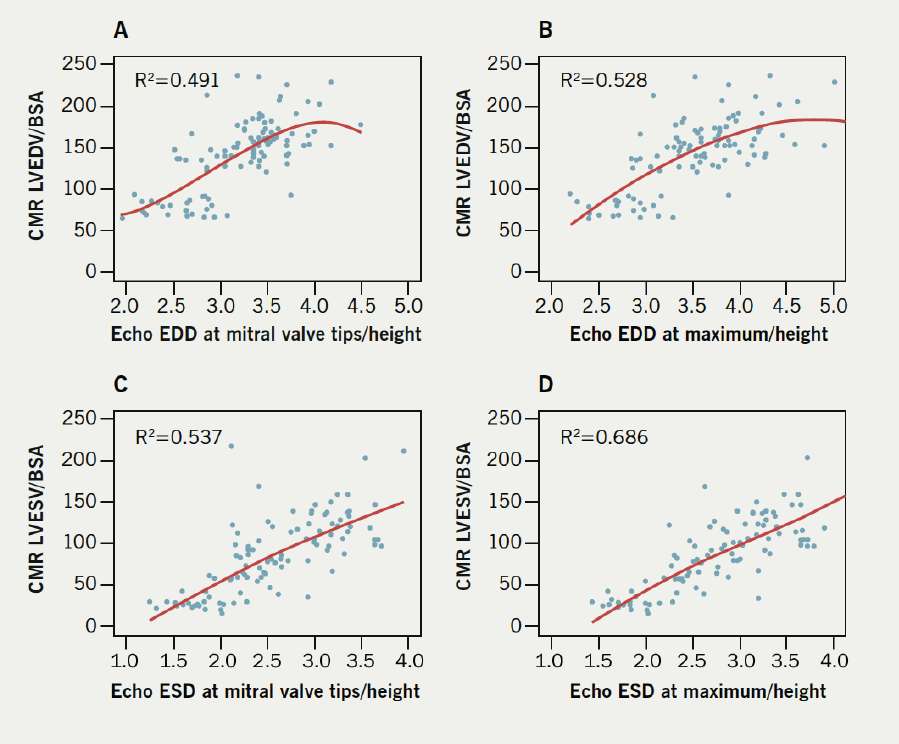
Indexed diameters at the maximum diameter exhibited better fits with LVEDV indexed by BSA when fitted to a cubic relationship. The R2 value for EDD at the maximum was 0.528, compared with 0.491 for EDD at the valve tips. For ESD, the maximum diameter showed a better fit as well (0.686 vs. 0.537) (figure 2).
When using standard cut-offs for absolute end-diastolic diameters on echocardiography (EDD >5.9 cm for males, >5.3 for females),3 diameters at mitral valve tips correctly identified 57.3% (47/82) of abnormal subjects, while incorrectly classifying 4.3% (1/23) of control subjects as abnormal. Absolute measurements at the maximum diameter identified 78.0% (64/82) of abnormal subjects and incorrectly classified 13.0% (3/23) controls. Using indexed end-diastolic diameters on echocardiography (EDDi >3.2 cm/m for males, >3.1 cm/m for females),3 diameters at mitral valve tips correctly identified 72.0% (59/82) of abnormal subjects and incorrectly classified 4.3% (1/23) of control subjects. Indexed measurements at the maximum diameter identified 87.8% of abnormal subjects and incorrectly classified 13.0% (3/23) of control subjects.
Discussion
In volume overload states such as AR, ICM, and NICM, CMR depicts spherical remodelling as a common remodelling pathway, an adaptation that maintains normal net forward stroke volume. These geometric alterations affect the assumed relationship between the standard LV dimensions measured at mitral valve leaflet tips and true LV volume. The results in this investigation demonstrate that EDDmax/ EDDMV and ESDmax/ESDMV ratios are larger in subjects with AR, ICM and NICM, where spherical dilation occurs. The percentage difference among these subjects did not vary with indexed LV volumes, indicating that the standard measurement at mitral valve tips underestimates LV size in spherical dilation regardless of the extent of remodelling. The maximum diameter on echocardiography showed a better fit to the presumed cubic relationship between diameter and volume than the diameter at mitral valve tips during diastole and systole. Although abnormal cut-offs for the maximum diameter are undefined, applying the same thresholds used for standard measurements to the maximum diameter improved sensitivity for detecting abnormal chamber volumes without substantially increasing the false-positive rate. Indexed dimensions demonstrated better classification of the presence or absence of LV dilation compared with absolute dimensions.
Limitations of the study include its retrospective nature, subjects being from a single tertiary care centre, and selection bias. The majority of cardiomyopathy patients had ischaemic damage, limiting the characterisation of remodelling in NICM, and there were relatively few control subjects for analysis. However, this is typical in a population where CMR is clinically indicated.
Spherical dilation is observed in AR, ICM and NICM. Indexed dimensions demonstrated better classification accuracy compared with absolute dimensions. Despite guidelines that refer to measurements at mitral valve leaflet tips, these results suggest that measuring the LV maximum dimension may better estimate LV volume in subjects with remodelling. A combination of both measurements may more accurately characterise LV volume and geometry, and the ratio of these measurements may serve as a straightforward method of quantifying the extent of spherical dilation.
Further investigations could verify the use of the maximum diameter as a stronger predictor of LV volume than the standard measurement in a larger cohort of patients with a greater cohort of control subjects. In addition, characterisation of the typical ranges of maximum diameters in relation to the standard measurement in various disease states both on CMR and echocardiography could be performed to determine the use of the ratio of these two measurements to determine the extent of remodelling, and the feasibility of incorporating the maximum diameter into clinical management guidelines.
Conflict of interest
All authors have no conflicts of interest to disclose.
Funding information
This work was supported by a National Institutes of Health training grant (T35 HL082544) for C.W.H.
Key messages
- In spherical remodelling, a single-dimensional measurement at the mitral valve leaflet tips may underestimate ventricular volume due to altered geometry
- Diameters measured at the widest dimension were more predictive of left ventricle (LV) volume than the standard measurements
- When using standard cut-offs, maximum diameters also improved categorisation of subjects with chamber enlargement without substantially increasing the false-positive rate
- Measuring the maximum diameter may be a straightforward approach to improve assessment of LV volume
References
1. American College of Cardiology Foundation Task Force on Expert Consensus Documents, Hundley WG, Bluemke DA et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol 2010;55:2614–62. http://dx.doi.org/10.1016/j.jacc.2009.11.011
2. Khouri MG, Peshock RM, Ayers CR, de Lemos JA, Drazner MH. A 4-tiered classification of left ventricular hypertrophy based on left ventricular geometry: the Dallas heart study. Circ Cardiovasc Imaging 2010;3:164–71. http://dx.doi.org/10.1161/CIRCIMAGING.109.883652
3. Lang RM, Bierig M, Devereux RB et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–63. http://dx.doi.org/10.1016/j.echo.2005.10.005
4. Bonow RO, Carabello BA, Chatterjee K et al. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:e1–e142. http://dx.doi.org/10.1016/j.jacc.2008.05.007
5. Maceira AM, Prasad SK, Khan M, Pennell DJ. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2006;8:417–26. Available from: http://www.scmr.org/assets/files/members/documents/JCMR/008/LCMR_i_008_03_tfja/LCMR_i_8_03_O/LCMR_A_157271_O.pdf
6. Grothues F, Smith GC, Moon JC et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002;90:29–34. http://dx.doi.org/10.1016/S0002-9149(02)02381-0
7. Lauer MS, Anderson KM, Larson MG, Levy D. A new method for indexing left ventricular mass for differences in body size. Am J Cardiol 1994;74:487–91. http://dx.doi.org/10.1016/0002-9149(94)90909-1
