The coagulation cascade
The function of the coagulation cascade is to generate fibrin (see figure 3). This happens via a sequence of highly regulated enzyme/substrate reactions,2 which occur largely on the surface of endothelial cells and platelets. Although traditionally divided into an ‘extrinsic’ pathway (tissue-factor dependent) and an ‘intrinsic’ pathway (contact factor dependent), it is currently thought that the tissue factor pathway is the main mechanism for haemostasis in vivo. The physiological role for the components of the ‘intrinsic’ pathway are less clear – factor XII deficiency, for example, does not seem to be associated with a bleeding phenotype. However, the division into ‘extrinsic’ and ‘intrinsic’ pathways is still useful in the interpretation of coagulation tests: broadly speaking, the prothrombin time (PT) assesses the extrinsic pathway, and the activated partial thromboplastin time (APTT) the intrinsic pathway.

Initiation
Tissue damage leads to exposure of blood to tissue factor, which complexes with factor VIIa. This complex has the ability to convert small amounts of factor X to factor Xa, and also IX to IXa.
Amplification
Factor Xa, generated in the initiation phase, can, in turn, generate a small amount of thrombin from prothrombin. This thrombin then acts on factors V and VIII (generating Va and VIIIa), and also and also converts XI to XIa which increases production of IXa from IX. VIIIa and IXa form a complex – the ‘tenase’ complex – which massively increases production of Xa from X. This increased amount of Xa complexes with Va to form the ‘prothrombinase complex’, which produces much more thrombin from prothrombin than Xa could alone. Thus by a sequence of amplification steps, there is an explosive generation of thrombin, which has a central role in production and stabilisation of a clot. The ‘tenase’ and ‘prothrombinase’ complex are assembled on phospholipid surfaces – hence the requirement for activated platelets.
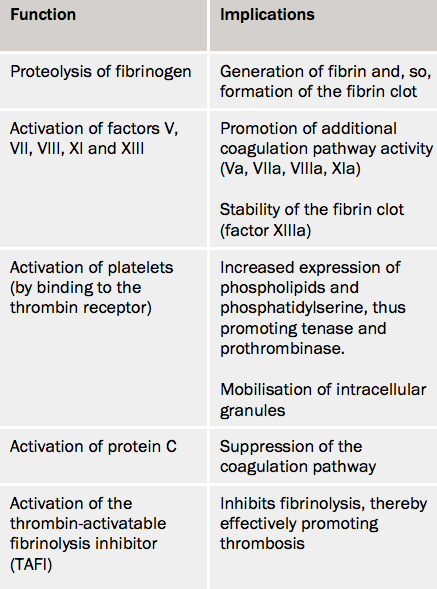
Formation of fibrin clot – the central role of thrombin
Thrombin cleaves fibrinogen to create fibrin monomers, which then spontaneously polymerise before being stably cross-linked by factor XIIIa (see figure 4).
Thrombin also has a number of other functions (see table 3): positive feedback and amplication of the coagulation cascade, platelet activation, inhibition of fibrinolysis, and activation of factor XIII. The central role of thrombin in coagulation has made it an attractive target for anticoagulant drugs (see module 3 for a full discussion of direct thrombin inhibitors). Thrombin generation has also attracted interest as a laboratory measure of global haemostatic function, which may have future clinical applications.3
Inhibitors
Coagulation inhibitors ensure the coagulation pathway does not develop too rapidly or too extensively. The primary regulator is antithrombin, which, in addition to inhibiting thrombin, also suppresses factors VIIa, IXa, Xa and XIa. However, in itself, antithrombin is a relatively weak inhibitor: 90% of its bioactivity is accounted for by its binding to heparin on the surface of the endothelium.
Protein C is a vitamin-K dependent zymogen, or pro-enzyme, or inactive enzyme precursor. The endothelial membrane component, thrombomodulin, binds thrombin, and in this form converts protein C to an active molecule (hence, activated protein C) that inhibits factors Va and VIIa and, to a lesser extent, thrombin. This activation is enhanced by the binding of protein C to the endothelial protein C receptor, and is stabilised by the presence of protein S. Tissue factor pathway inhibitor (TFPI) suppresses the activity of tissue factor in the initiation of the cascade.
