Summary – formation of a platelet plug in response to injury1,4,5
Although platelets and the coagulation cascade work together to secure haemostasis, formation of a platelet plug is arguably the most important mechanism behind securing primary haemostasis in conditions of high shear stress, such as in arterial injury. Considering the steps involved provides a useful summary of platelet recruitment and activation:
(i) Initial platelet tethering. Damage to the vessel wall leads to exposure of collagen, which binds von Willebrand factor. The latter then binds platelets via GpIb/IX/V.
(ii) Stable adhesion and activation. Stable adhesion at the site of injury is mediated through receptors including GpVI and GpIIb/IIIa. GpIIb/IIIa also binds fibrinogen and other platelets via fibrinogen, increasing platelet recruitment. Binding of receptors leads via intracellular signalling to platelet activation, and resultant shape change, granule release and thromboxane A2 generation.
(iii) Spreading and aggregation. Positive feedback mechanisms described above lead to further platelet recruitment. Platelet conformational change exposes phosphatidylserine, providing procoagulant surface for optimal function of components of coagulation cascade. Activated platelets become stably cross-linked (aggregation). Activation of coagulation cascade generates thrombin which stabilises the platelet plug.
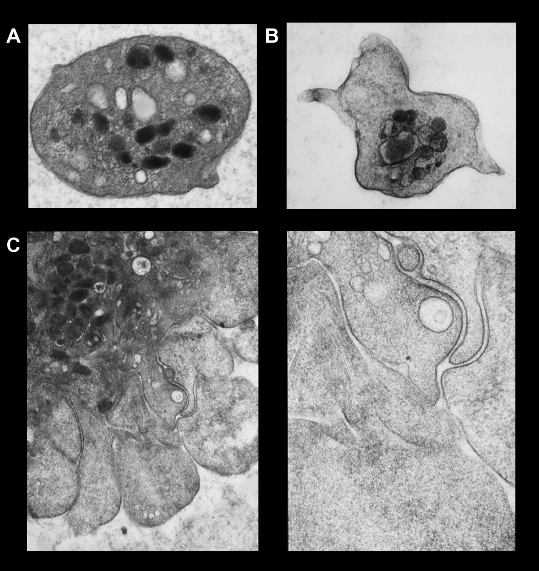
The transition from inactive platelet to active can be seen under the electron microscope in figure 5. As an introduction to pharmacological intervention in platelet function, Figure 66 shows a stylised view of platelet activation and the targets of various antiplatelet agents.

Fibrinolysis
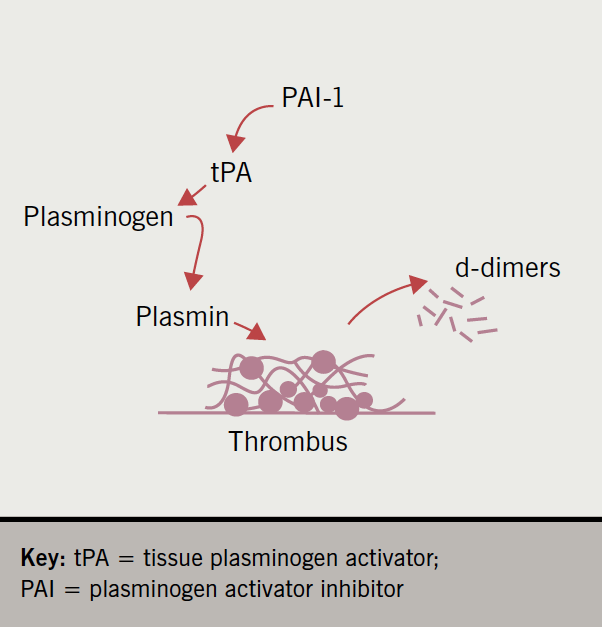
Once a thrombus has done its job and reduced blood loss, and the damage is repaired, it must be removed. This is performed largely by the enzyme plasmin in the process of fibrinolysis, sometimes expressed as thrombolysis. Plasmin itself is derived from the zymogen plasminogen by the action of tissue plasminogen activator (tPA), which is an endothelial product.
However, tPA has a regulator, plasminogen activator inhibitor, of which there are several types (hence PAI-1). Plasminogen activator inhibitor can be released from platelets, endothelial cells and other cells. Since tPA and PAI-1 are believed to react in a stoichiometry of 1:1, the balance between the two is crucial for the process of fibrinolysis.
When plasmin breaks down cross-linked fibrin, it generates quite specific protein fragments that are easily identified in the plasma. These fragments are called d-dimers, and high levels are considered proof of active fibrinolysis, and, therefore, of a high general burden of thrombus within the body. This process is summarised in figure 7.
The dynamics of haemostasis
An old view of haemostasis considered it to be a ‘stop–start’ model, where various factors would initiate the process, which would proceed and eventually stop with the formation of a clot. In the light of new research, this view has been superseded by the dynamic hypothesis.
In the dynamic model, the coagulation system is permanently active, but at a low level, and is held in check by inhibitors. The platelet pool is at rest and very few are activated. Upon stimulation, coagulation activity increases, escapes from inhibitor regulation, and thrombus formation follows. However, the inhibitors soon catch up and eventually coagulation activity slows down, which prevents the process from expanding too rapidly. Quite possibly in parallel, platelets are activated (by thrombin for example), degranulate, and so promote the coagulation pathway. The platelet shape change favours adhesion and aggregation, and binding to fibrin results in thrombus formation.
This model predicts that there are always background levels of active factors generating a small amount of clot, but that this is degraded by low levels of plasmin generated from the fibrinolysis pathway. Indeed, the small amounts of plasma d-dimers present in healthy blood support this hypothesis.
Thus, the balance between a semi-active coagulation system, regulation by inhibitors, and fibrinolysis is crucial in thrombosis. The attraction and strength of this model is that it explains many of the causes of clinical thrombosis, predicts outcomes, and provides opportunities to intervene.
