Introduction
Transcatheter approaches to the mitral and aortic valves offer an alternative therapeutic strategy currently reserved for patients with a predicted high surgical mortality. This module focuses on recently approved devices, as well as investigational approaches to mitral and aortic valve reconstruction.

The background to transcatheter aortic valve implantation (TAVI) was recently reviewed in a TAVI-focus issue of the European Heart Journal.1 In a search for less invasive treatment methods, the concept of transcatheter implantation of heart valves was pioneered by Henning Anderson for the aortic, and Philipp Bonhoffer for the pulmonary position, followed by the first percutaneous human implantation of an aortic valve prosthesis by Alain Cribier in 2002. The Food and Drug Administration (FDA) approved the SAPIEN valve in November 2011 for the treatment of inoperable patients, and expanded the indication to high-risk surgical patients in October 2012. Over the past decade the treatment of severe aortic stenosis has been revolutionised with over 10,000 TAVI procedures being performed.
Aortic valve replacement
The basic concept of the transcatheter aortic valve replacement devices involves delivering a stented trileaflet bioprosthetic valve to the site of the diseased native valve, either via a transfemoral, transapical, or direct aortic approach (see figure 1).
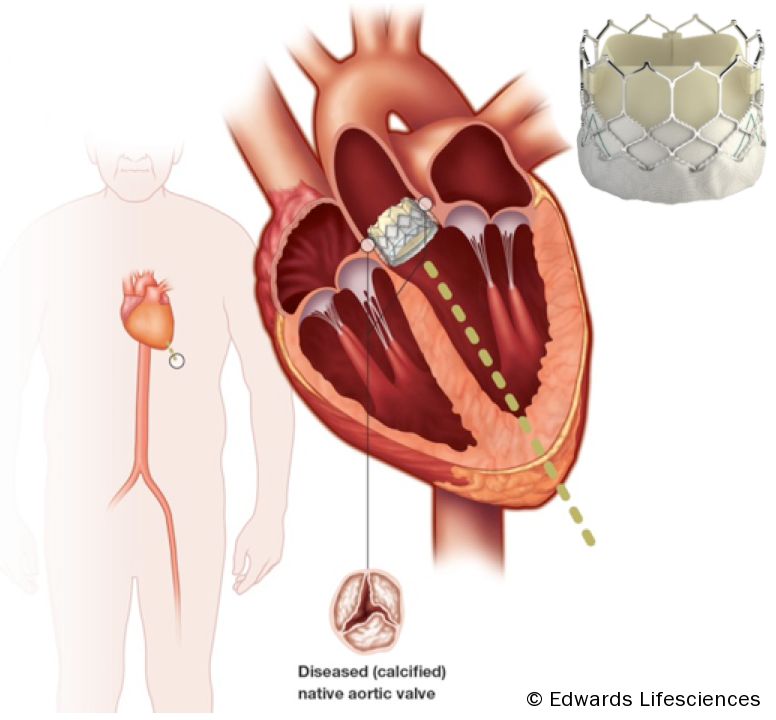
Patient selection
TAVI is indicated for symptomatic severe aortic stenosis when the ‘heart team’ at a cardiac surgical centre has determined that the patient:
- Is unsuitable for conventional surgery because of severe comorbidities
- Is likely to have an improvement in quality of life as a result of the intervention
- Has a life expectancy of at least one year.
The calculation of risk is difficult since no wholly satisfactory score exists. A combination of surgical scores and a frailty index should be used and it is important to have specialists in elderly care involved in the decision.
If the estimated surgical risk is not high, the heart team may still decide that TAVI is preferable to conventional surgery for individual reasons including frailty, prior chest irradiation, porcelain aorta. Its use in intermediate-risk and lower risk patients is discussed later.
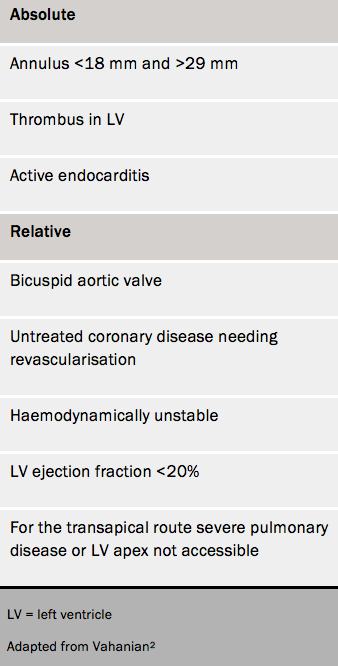
Contraindications for TAVI are given in table 1.2,3
