Evidence for TAVI
The risks and benefits for catheter-based aortic valve procedures versus open aortic valve replacement or non-operative therapy have been defined in two important trials. The multicenter randomised PARTNER (Placement of Aortic Transcatheter Valves) trials are the pivotal studies in the USA. In the first trial, 358 patients with severe aortic stenosis who were not candidates for surgery were randomised to two groups: the standard non-operative therapy (which included balloon valvuloplasty) or transcatheter implantation of a balloon-expandable valve.
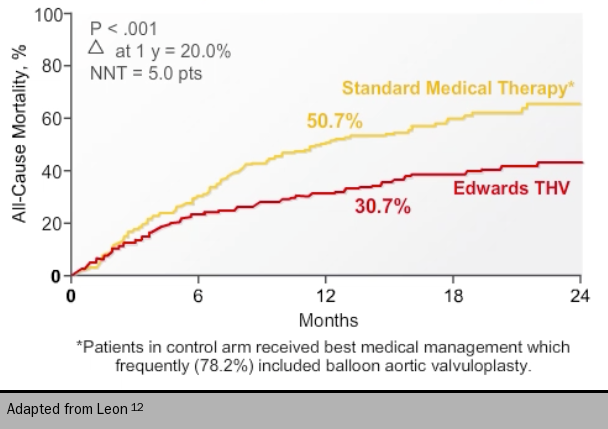
Rate of death from any cause at one year, the primary end point, was lower in the transcatheter implantation group than the standard therapy group (30.7% and 50.7%, respectively) (see figure 712). Cardiac symptoms at one year, defined as NYHA class III or IV, occurred in 25% of the transcatheter implantation group versus 58% in the standard therapy group.12 Their next trial and its two-year follow-up showed the transcatheter approach was not inferior to open aortic valve replacement in high-risk surgical patients.
The trial involved 699 patients and showed that the rates of death at two years were similar in both groups (33.9% and 35.0%) and reduction in symptoms was also similar. However, major vascular complications and paravalvular regurgitation were higher in the transcatheter implantation group. Also, stroke rates at 30 days were higher in the transcatheter implantation group (4.6% and 2.4%), but rates at two years were similar (37.1% and 36.4%).13
Three-year data (see table 2) from the PARTNER trial were presented at the American College of Cardiology (ACC) 62nd Annual Scientific Session in San Francisco (March 2013).
At three years, all-cause mortality for patients treated with the SAPIEN aortic valve implantation (TAVI) was statistically equivalent to that of patients who had received open-heart surgical aortic valve replacement (AVR). Symptom improvement and valve performance was similar in both groups and was maintained through the three years of patient follow-up. The incidence of stroke between TAVI and surgery patients was also comparable.
The PARTNER Trial is the first randomised, controlled trial of a transcatheter aortic valve in the USA. The high-risk surgery cohort (Cohort A) of the trial enrolled between May 2007 and Sept. 2009 and studied 699 patients with severe, symptomatic aortic stenosis deemed at high risk for traditional open-heart surgery. Patients were evaluated by a multi-disciplinary Heart Team and randomised to receive either traditional open-heart surgery or the Edwards SAPIEN valve with transfemoral or transapical delivery.
