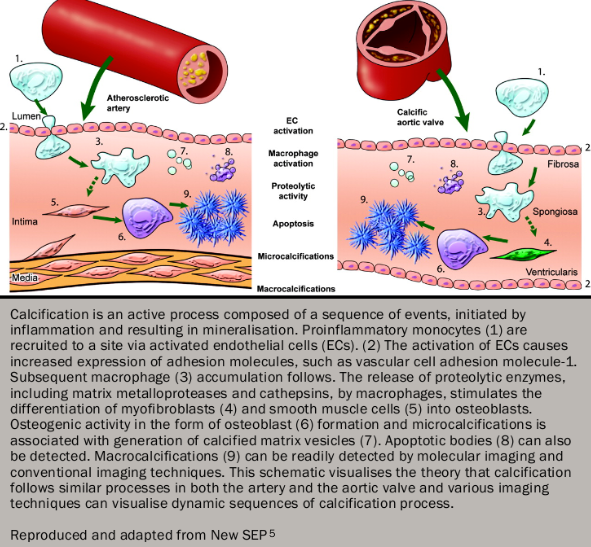
Initiation phase
Many of the same lipoproteins involved in atherogenesis (e.g. low-density lipoprotein and lipoprotein a) are also involved in the early stages of aortic stenosis and undergo oxidative modification. Inflammation predominantly consists of macrophages and T helper cells, which secrete pro-inflammatory cytokines including transforming growth factor β1, tumour necrosis factor and interleukin 1β. These subsequently drive the differentiation of fibroblasts: first into myofibroblasts and then into osteoblasts. These two cell-types co-ordinate the increased fibrosis and calcification within the valve, which are responsible for the increased leaflet thickening and stiffness. Fibrosis predominantly consists of types I and III collagen, which are secreted by myofibroblasts under the influence of the renin-angiotensin system. This occurs alongside the action of matrix metalloproteinases, which have a key role in restructuring the existing extracellular matrix.
Propagation phase
Calcification appears to be the predominant process driving disease progression in calcific degenerative and bicuspid valves. In the early stages, micro-calcification can be observed co-localising to areas of inflammation and lipid deposition. Once osteoblast differentiation has occurred, however, calcification occurs by means of a highly regulated process akin to skeletal bone formation with many of the same regulatory proteins implicated. Initially calcific nodules develop on the aortic aspect of the valve comprising hydroxyapatite deposited on a matrix of collagen, osteopontin and other bone matrix proteins. As aortic stenosis progresses, remodelling of this calcification occurs until by the later stages of disease, lamellar bone, microfractures and haemopoeitic tissue can all be identified within the valve (figure 45).
Other forms of calcific disease
Calcification of the mitral valve annulus is also a frequent finding, particularly in the elderly. Its pathophysiology is believed to share many similarities with calcific aortic valve disease. It too is associated with cardiovascular risk factors and believed to be triggered by the effects of increased mechanical stress. In the vast majority of patients, it has little effect on the function of the mitral valve but it has been demonstrated to be an independent risk factor for cardiovascular events and, with an ageing population, is becoming an increasingly common cause of stenosis and regurgitation.
Calcification is also the predominant process driving degeneration of bioprosthetic tissue valves. Most commonly these valves are comprised of porcine or bovine pericardial tissue sterilised with glutaraldehyde and mounted on a frame. Calcific degeneration can lead to cusp tears with regurgitation, embolic events or progressive stenosis with valve failure usually occurring between 10 and 15 years after their insertion. Whilst this may represent an immune reaction to the leaflet preparation, increasing evidence has suggested that the calcific mechanisms are similar to those observed in native valve disease.
