An additional factor affecting right ventricular afterload is the increased stiffness of the pulmonary circulation brought about by pulmonary vascular disease. The changes in the pulmonary circulation are brought about by vasoconstriction in the early stages of the disease, intimal cell proliferation into the lumen of the vessel and thrombosis. The cell proliferation is the most important part of the pathophysiology in many patients, and marks this vascular disease out from atherosclerosis in the systemic circulation.
The consequences for the right ventricle depend on its ability to adapt to these changed pulmonary haemodynamics. This depends on:
- age of onset of the disease
- rate of rise of PVR
- the genetic and phenotypic features of the right ventricle, much of which is as yet unknown.
The right ventricle dilates and hypertrophies: right ventricular dilatation results in worsening tricuspid regurgitation because the tricuspid valve annulus is stretched and the valve leaflets become unable to coapt. Tricuspid regurgitation adds volume overload to the already pressure-overloaded right ventricle, setting up a vicious cycle of restricted flow through the large and intermediate-sized pulmonary arteries and arterioles causing an increase in PVR and eventually death due to right heart failure.
Lung biopsies are used infrequently in the diagnosis of PH (they are usually contra-indicated owing to high risk of significant morbidity or mortality) but abnormalities seen at biopsy include intimal hyperplasia and medial and adventitial hypertrophy and hyperplasia.4 The underlying pathogenesis of these histological changes is one of endothelial cell dysfunction, excessive smooth muscle cell proliferation and reduced rates of apoptosis along with infiltration and incorporation of fibroblasts from surrounding parenchyma into the vessel wall. This occurs in the distal, previously unmuscularised, pulmonary arterioles and ultimately leads to loss of the vascular luminal cross-section.5 In advanced stages, a network of capillaries is often found adjacent to diseased vessels. These networks are called “plexiform” lesions and are thought to arise as a consequence of a monoclonal proliferation of endothelial cells.
The response of the right ventricle to the increased PVR is variable, for reasons that are not understood, but right ventricular function is critical in determining the patient’s prognosis. The right ventricle is more tolerant of raised PVR in children, as seen in children who have PAH in association with congenital heart disease.
Molecular changes
The vascular remodelling seen in PAH may be a consequence of genetic factors, endothelial dysfunction altering the vasoconstrictor/vasodilator balance and inflammatory changes involving chemokines, cytokines and growth factors.6 These changes, the understanding of which is rapidly evolving, are beyond the scope of this handbook and are well described in contemporary review articles.
Endothelial dysfunction leads to chronically impaired production of vasodilator and antiproliferative substances such as nitric oxide (NO) and prostacyclin, along with overexpression of vasoconstrictor and proliferative substances including thromboxane A2 and endothelin-1.2 All these substances are open to therapeutic manipulation (see the Treatments section of the handbook). The neuropeptide vasodilator vasoactive peptide (VIP), which has a pharmacological profile similar to prostacyclin, is also present in reduced quantities in the lungs of patients with PH.
In PAH, the balance between prostacyclin and thromboxane A2 is shifted towards the latter, favouring thrombosis, proliferation and vasoconstriction.7 Prostacyclin synthase is also decreased in pulmonary arteries in PAH. NO is a vasodilator and inhibitor of platelet activation and smooth muscle cell proliferation. In patients with PAH, both decreased levels of expression and increased levels of inhibition of endothelial nitric oxide synthase have been found.
Endothelin-1 (ET-1) is a potent vasoconstrictor and stimulant of smooth muscle cell proliferation in pulmonary arteries, acting via the endothelin A and B receptors. Plasma levels of ET-1 are raised in PAH, and correlate with the severity of disease and with the prognosis.8
Other abnormalities observed in smooth muscle cells in PAH (which favour a decreased apoptosis/proliferation ratio) include inappropriate activation of transcription factors HIF-1 alpha and NFAT, decreased expression of potassium channels Kv1.5 and Kv2.1 and expression of the antiapoptotic protein survivin.9
Prevalence of pulmonary hypertension
The epidemiology of pulmonary hypertension is not well described although registries are now rectifying this situation. In the UK 2014 National Audit of Pulmonary Hypertension, PAH was the most common diagnosis, occurring in 45% of patients, followed by chronic thromboembolic pulmonary hypertension (CTEPH) in 20%. The main causes of PAH were congenital heart disease (34%), idiopathic, heritable or anorexigen-induced (33%) and connective tissue disease (23%).25 A prevalence of 97 cases per million with a female:male ratio of 1.8 has also been reported.1 Traditionally, the mean age of patients with PAH has been thought to be 36, stemming from the first US National Institutes of Health registry in the 1980s. However, in current registries, the mean age at diagnosis is between 50–65 years. In the UK, the median age for females is 60 and for males is 58.25
PH due to left heart disease (group 2) and lung disease/hypoxia (group 3) are an important part of clinical practice, though there is little information about the demographics and clinical course. Up to 60% of patients with severe left ventricular (LV) systolic dysfunction and up to 70% of patients with heart failure with preserved ejection fraction may present with PH. Most patients with severe symptomatic mitral valve disease will have PH and up to 65% with symptomatic aortic stenosis.1
CTEPH prevalence in the UK has been estimated to be between 8 and 40 cases per million. The incidence of CTEPH after an acute PE is in the range of 0.5–2%. A history of acute PE was reported for 74.8% of patients from the International CTEPH Registry.1 Globally, schistosomiasis associated PAH and high-altitude related PH are important causes.
Functional classification of pulmonary hypertension
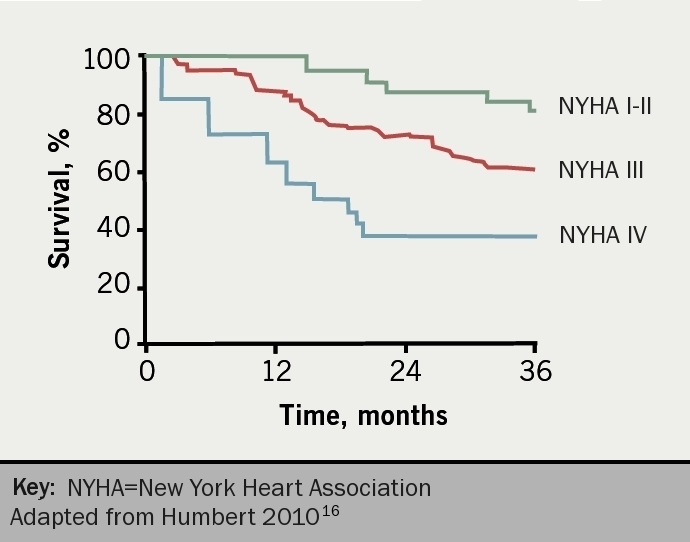
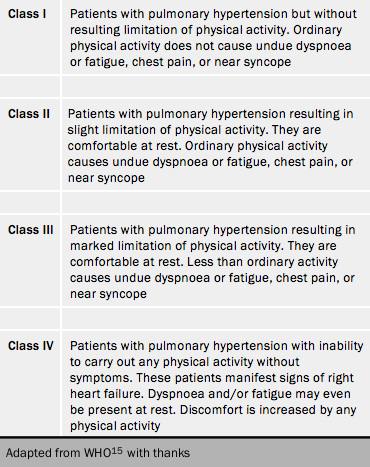
The severity of PH is assessed according to the functional classification modified after the New York Heart Association classification according to the World Health Organization (table 4).15 Functional class is a powerful predictor of survival in PH despite inter-observer variation (figure 5).16 It remains the case that most patients present to a PH centre in WHO FC III. This figure illustrates the consequence of waiting for symptoms to deteriorate before reaching a diagnosis. Syncope, which usually starts as exertional, is generally considered to put patients into functional class IV although this was never stated in the original statement.
