Dipeptidyl peptidase-4 (DPP-4) inhibitors are one of two classes of antidiabetes drugs that mediate their glucose-lowering effect through the incretin pathway. They are administered orally and offer significant glucose-lowering with a neutral weight profile and a low risk of hypoglycaemia. Three large randomised-controlled trials have demonstrated cardiovascular safety, with no increase in major adverse cardiovascular events comparing DPP-4 inhibitors (saxagliptin, alogliptin and sitagliptin) with placebo. An increase in heart failure hospitalisation was noted with saxagliptin compared with placebo, and a similar increase was also noted in one subgroup receiving alogliptin compared with placebo. Further cardiovascular safety trials with DPP-4 inhibitors are ongoing, including a trial comparing the DPP-4 inhibitor linagliptin with the sulphonylurea glimepiride.
Introduction
 Over the past decade, several new classes of glucose-lowering therapies have become available for the treatment of type 2 diabetes mellitus (T2DM). The need for evidence demonstrating cardiovascular safety of new diabetes therapies was highlighted in 2007 when it was suggested that treatment with rosiglitazone increased rates of myocardial infarction and possibly cardiovascular death.1 Following this controversy, the Food and Drug Administration (FDA) and European Medicines Agency (EMA) issued guidance to drug manufacturers requiring that they produce evidence to demonstrate any new therapy does not result in an ‘unacceptable increase’ in cardiovascular risk.2,3 Evidence should be collected through large clinical trial programmes involving patients at higher risk of cardiovascular events, both to ensure sufficient end points are detected, and so that there is a true representation of the type 2 diabetic population. Often this will include a dedicated cardiovascular outcome trial (CVOT) with a randomised design and a minimum of two years follow-up.
Over the past decade, several new classes of glucose-lowering therapies have become available for the treatment of type 2 diabetes mellitus (T2DM). The need for evidence demonstrating cardiovascular safety of new diabetes therapies was highlighted in 2007 when it was suggested that treatment with rosiglitazone increased rates of myocardial infarction and possibly cardiovascular death.1 Following this controversy, the Food and Drug Administration (FDA) and European Medicines Agency (EMA) issued guidance to drug manufacturers requiring that they produce evidence to demonstrate any new therapy does not result in an ‘unacceptable increase’ in cardiovascular risk.2,3 Evidence should be collected through large clinical trial programmes involving patients at higher risk of cardiovascular events, both to ensure sufficient end points are detected, and so that there is a true representation of the type 2 diabetic population. Often this will include a dedicated cardiovascular outcome trial (CVOT) with a randomised design and a minimum of two years follow-up.
This guidance has been applied in the development of the three major new classes of glucose-lowering medication used in T2DM: dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) agonists and sodium-glucose co-transporter 2 (SGLT2) inhibitors. Large randomised, placebo-controlled trials with dedicated cardiovascular outcomes have been undertaken with results now available for drugs within all three classes. These outcome studies have been eagerly anticipated by the diabetes and cardiology communities, with the hope that cardiovascular safety can be demonstrated alongside a glucose-lowering effect. Furthermore, the possibility that a drug may also offer a benefit in reducing cardiovascular outcomes, particularly one that could be demonstrated over a relatively modest follow-up period, is clearly an exciting prospect. The results of these trials, and others currently underway, will undoubtedly influence evidence-based prescribing practice for all clinicians involved in the management of patients with T2DM.
Pharmacology of DPP-4 inhibitors
DPP-4 inhibitors exhibit their action through augmenting the incretin effect. They act to increase the systemic concentration of GLP-1, one of two known incretin hormones, by preventing its breakdown by the enzyme DPP-4. GLP-1 is released from intestinal mucosal cells in response to food and has several physiological actions including promotion of insulin release, inhibition of glucagon release, delayed gastric emptying and increased satiety.
DPP-4 inhibitors have been shown to reduce HbA1c by around 0.7%.4 This is less marked than the reduction seen with GLP-1 agonists, however, they have the advantage of oral bioavailability. Other features include low rates of hypoglycaemia and a neutral weight profile. In the UK, there are five approved preparations: sitagliptin, vildagliptin, saxagliptin, linagliptin and alogliptin. They are renally excreted with the exception of linagliptin, which undergoes excretion by the biliary system. This allows for use of a single dose of linagliptin across the spectrum of renal function.
DPP-4 inhibitors are recommended by the Scottish Intercollegiate Guidelines Network (SIGN) and the National Institute for Health and Care Excellence (NICE) for use in T2DM as a second- or third-line oral agent, in addition to metformin and/or sulphonylurea.5,6 NICE also recommends dual therapy alongside pioglitazone or use as monotherapy in patients who cannot tolerate metformin or sulphonylurea.6 Prescribing considerations are shown in table 1.

SAVOR-TIMI 53
SAVOR-TIMI 53 (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus – Thrombolysis in Myocardial Infarction 53) assessed the cardiovascular safety of saxagliptin in a large, randomised, double-blind, placebo-controlled trial.7 It involved 16,492 patients with T2DM (HbA1c 6.5–12%) across 788 sites in 26 countries. Patients had a history of established cardiovascular disease (age ≥40 years and previous event relating to atherosclerotic disease involving the cardiac, cerebral or peripheral vascular system) or multiple risk factors for vascular disease (men age ≥55 years or women ≥60 years; at least one of dyslipidaemia, hypertension or current smoking). Baseline cardiovascular disease included investigator-diagnosed heart failure in 2,105 patients (13%). Patients were randomised to receive saxagliptin 5 mg daily (2.5 mg daily if estimated glomerular filtration rate [eGFR] ≤50 ml/min/1.73 m2) or placebo in a 1:1 ratio with a median follow-up duration of 2.1 years.
The primary end point was major adverse cardiovascular events (MACE), a composite of nonfatal myocardial infarction, nonfatal stroke and cardiovascular death. There was no statistically significant difference in this outcome between groups with 613 (7.3%) events in the saxagliptin group and 609 (7.2%) events in the placebo group (hazard ratio [HR] 1.00; 95% confidence interval [CI] 0.89 to 1.12; p<0.001 for noninferiority). Unexpectedly, there was a significant increase in the rate of hospitalisation for heart failure in the saxagliptin group with 289 (3.5%) events compared with 228 (2.8%) events in the placebo group (HR 1.27; 95% CI 1.07 to 1.51; p=0.007). Post-hoc analysis showed the significant increase occurred in patients taking saxagliptin with no previous history of heart failure (2.3% vs. 1.7%; HR 1.32; 95% CI 1.04 to 1.66; p=0.02).8 No significant difference in hospitalisation for heart failure was seen in patients with heart failure at baseline.
EXAMINE
EXAMINE (Examination of Cardiovascular Outcomes With Alogliptin versus Standard of Care) was a double-blind trial that randomised type 2 diabetic patients in a 1:1 ratio to receive alogliptin or placebo following an acute coronary syndrome (acute myocardial infarction or unstable angina requiring hospitalisation).9 The study recruited 5,380 patients (baseline HbA1c 8.0 ± 1.1%) from 898 centres in 49 countries. Treatment was commenced within 15 to 90 days of the acute coronary syndrome for a median duration of 18 months. Alogliptin was administered in three dose increments (25 mg, 12.5 mg or 6.25 mg daily) based on renal function at baseline. Heart failure was present in 1,501 patients (28%) at baseline, and pre-dated the index acute coronary syndrome event in approximately 60% of cases.9,10
The primary MACE end point (comprising cardiovascular death, nonfatal myocardial infarction and nonfatal stroke) occurred in 305 (11.3%) patients in the alogliptin group and 316 (11.8%) patients in the placebo group (HR 0.96; p<0.001 for noninferiority). Surprisingly, EXAMINE did not report on heart failure outcomes in the primary publication. Following the publication of SAVOR-TIMI 53, however, post-hoc analysis was published.10 Overall, heart failure hospitalisation occurred in similar numbers in the alogliptin and placebo groups with 106 and 89 events, respectively (HR 1.19; 95% CI 0.90 to 1.58; p=0.220). However, subgroup analysis of patients with no history of heart failure at baseline showed alogliptin was associated with an increased risk of heart failure hospitalisation with 43 (2.2%) events in the alogliptin group compared with 24 (1.3%) events in the placebo group (HR 1.76; 95% CI 1.07 to 2.90; p=0.026). There was no significant difference seen for patients with heart failure at baseline.
TECOS
TECOS (Trial to Evaluate Cardiovascular Outcomes after Treatment with Sitagliptin) examined the cardiovascular safety of sitagliptin in patients with T2DM and existing cardiovascular disease.11 There were 14,671 patients (HbA1c 7.2 ± 0.5%) randomised in a 1:1 ratio to receive sitagliptin 100 mg daily (50 mg daily if eGFR ≥30 and <50 ml/min/1.73 m2) or placebo. Established cardiovascular disease was defined as major coronary artery disease, cerebrovascular disease or peripheral arterial disease. This trial had a longer median follow-up period of three years and collected data from 673 centres across 38 countries. Baseline characteristics included heart failure in 2,643 (18%) participants.
The primary end point (MACE plus – a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke and hospitalisation for unstable angina) occurred in 839 (11.4%) patients receiving sitagliptin and 851 (11.6%) patients receiving placebo (HR 0.98; 95% CI 0.88 to 1.09; p<0.001 for noninferiority). Heart failure hospitalisation occurred in similar numbers in sitagliptin and placebo groups with 228 (3.1%) and 229 (3.1%) events, respectively (HR 1.00; 95% CI 0.83 to 1.20; p=0.98). A composite outcome of heart failure hospitalisation and cardiovascular death also did not differ significantly between groups with 538 (7.3%) events in the sitagliptin group and 525 (7.2%) events in the placebo group (HR 1.02; 95% CI 0.90 to 1.15; p=0.74). Further analysis comparing subjects with heart failure at baseline showed no significant difference in hospitalisation for heart failure with 97 (7.4%) events in the sitagliptin group and 94 (7.0%) events in the placebo group (HR 1.03; 95% CI 0.77 to 1.36; p=0.86).12 In this subgroup, there was also no significant difference in cardiovascular death when comparing sitagliptin and placebo groups (9.2% vs. 9.9%; HR 0.91; 95% CI 0.71 to 1.17; p=0.46).
Discussion
DPP-4 inhibitors have demonstrated cardiovascular safety, in terms of no increase in atherosclerotic events, in patients with T2DM and existing cardiovascular disease. This observation is also supported in a meta-analysis of vildagliptin versus comparators where no increased risk of MACE end points was seen.13 The failure of DPP-4 inhibitors to demonstrate any cardioprotective benefit may relate to their minimal effects on weight, blood pressure and lipid profile, however, their long-term effects beyond study follow-up are unknown. Table 2 summarises CVOT data for DPP-4 inhibitors.
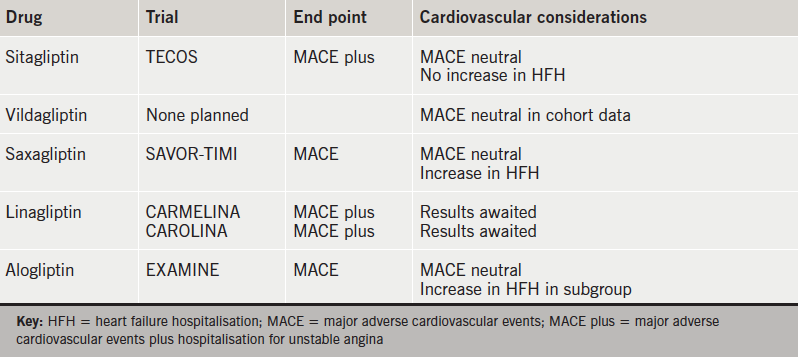
The increased heart failure hospitalisation seen in SAVOR-TIMI 53 was unexpected by the authors. Interpretation within the context of multiple testing was advised and an underlying mechanism for the increase in hospitalisation was not hypothesised.7 DPP-4 has multiple peptide substrates involved in cardiovascular function, including brain natriuretic peptide (BNP), substance P and neuropeptide Y.14 Studies involving porcine models and human cardiomyocytes and fibroblasts have shown inconsistent results with regards to the biological effects of BNP before and after cleavage by DPP-4.15 Therefore, the relationship between DPP-4 inhibitors, BNP and heart failure is uncertain and further study is required. Results are awaited from two large cardiovascular safety studies comparing linagliptin to placebo and the sulphonylurea glimepiride.16,17
Conflict of interest
EJ: none declared. GMcK has received payments for lectures and advisory boards from Astra Zeneca, Boehringer Ingelheim and MSD. MF has received payment for lectures and advisory boards from Astra Zeneca, Boehringer Ingelheim, MSD, Novartis and Takeda.
Editors’ note
Subsequent articles in this series will discuss SGLT2 inhibitors (doi:10.5837/bjc.2017.010), glitazones (thiazolidinediones) (doi:10.5837/bjc.2017.018), Glucagon-like peptide-1 (GLP-1) receptor agonists (doi:10.5837/bjc.2017.030) Older antidiabetic drugs (doi: 10.5837/bjc.2018.007) and glucose-lowering drugs for patients with cardiac disease (doi:10.5837/bjc.2018.016).
Key messages
- Dipeptidyl peptidase-4 inhibitors (DPP-4 inhibitors) improve glycaemic control in type 2 diabetes mellitus by inhibiting the breakdown of glucagon-like peptide-1 (GLP-1), therefore, increasing glucose-dependent insulin production
- Three DPP-4 inhibitors (saxagliptin, alogliptin and sitagliptin) demonstrated no increase in major adverse cardiovascular events when compared with placebo in large randomised-controlled trials (RCTs)
- Heart failure hospitalisation was increased for patients receiving saxagliptin compared with placebo, and also in a subgroup of patients receiving alogliptin compared with placebo
- The cardiovascular safety of linagliptin is being examined in two RCTs expected to report by 2020.
References
1. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–71. https://doi.org/10.1056/NEJMoa072761
2. Food and Drug Administration. Guidance for industry. Diabetes mellitus – evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), 2008. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071627.pdf [accessed 17 September 2016].
3. European Medicines Agency. Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. London: EMA, 2012. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/06/WC500129256.pdf [accessed 17 September 2016].
4. Monami M, Iacomelli I, Marchionni N, Mannucci E. Dipeptydil peptidase-4 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis 2010;20:224–35. https://doi.org/10.1016/j.numecd.2009.03.015
5. Scottish Intercollegiate Guidelines Network (SIGN). Management of diabetes: a national clinical guideline. Edinburgh: SIGN, 2010. Available from: http://www.sign.ac.uk/pdf/sign116.pdf [accessed 17 September 2016].
6. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. London: NICE, 2015. Available from: https://www.nice.org.uk/guidance/ng28/resources/type-2-diabetes-in-adults-management-1837338615493 [accessed 17 September 2016].
7. Scirica BM, Bhatt DL, Braunwald E et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317–26. https://doi.org/10.1056/NEJMoa1307684
8. Scirica BM, Braunwald E, Raz I et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation 2014;130:1579–88. https://doi.org/10.1161/CIRCULATIONAHA.114.010389
9. White WB, Cannon CP, Heller SR et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes for the EXAMINE investigators. N Engl J Med 2013;369:1327–35. https://doi.org/10.1056/NEJMoa1305889
10. Zannad F, Cannon CP, Cushman WC et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: A multicentre, randomised, double-blind trial. Lancet 2015;385:2067–76. https://doi.org/10.1016/S0140-6736(14)62225-X
11. Green JB, Bethel MA, Armstrong PW et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:232–42. https://doi.org/10.1056/NEJMoa1501352
12. McGuire DK, Van de Werf F, Armstrong PW et al. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol 2016;1:126–35. https://doi.org/10.1001/jamacardio.2016.0103
13. McInnes G, Evans M, Del Prato S et al. Cardiovascular and heart failure safety profile of vildagliptin: a meta-analysis of 17 000 patients. Diabetes Obes Metab 2015;17:1085–92. https://doi.org/10.1111/dom.12548
14. Standl E, Erbach M, Schnell O. Dipeptidyl-peptidase-4 Inhibitors and heart failure: class effect, substance-specific effect, or chance effect? Curr Treat Options Cardiovasc Med 2014;16:353. https://doi.org/10.1007/s11936-014-0353-y
15. Vanderheyden M, Bartunek J, Goethals M et al. Dipeptidyl-peptidase IV and B-type natriuretic peptide. From bench to bedside. Clin Chem Lab Med 2009;47:248–52. https://doi.org/10.1515/CCLM.2009.065
16. Clinicaltrials.gov. Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus (CARMELINA). Available from: https://clinicaltrials.gov/ct2/show/NCT01897532 [accessed 21 September 2016].
17. Marx N, Rosenstock J, Kahn SE et al. Design and baseline characteristics of the CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes (CAROLINA(R)). Diabetes Vasc Dis Res 2015;12:164–74. https://doi.org/10.1177/1479164115570301
