The theme running through the recent 2017 British Cardiovascular Society (BCS) Annual Conference was ‘Cardiology at the extremes’. Few conditions in medicine can be more extreme than chronic and acute exacerbations of heart failure. In this report from the BCS meeting in Manchester, we focus on heart failure since it is 30 years since the landmark Co-operative North Scandinavian Enalapril Survival Study (CONSENSUS) was reported, a milestone noted at the conference. In accompanying podcasts, we interview some of the presenters who have been much involved in heart failure services in the UK to find out their experiences of the ‘real world’ of managing heart failure outside randomised clinical trials and also consider what challenges remain in this field.
Landmark trials in heart failure – 30 years from CONSENSUS
With 2017 marking the 30th year since the publication of CONSENSUS,1 which first reported a reduction in mortality with enalapril versus placebo in patients with advanced heart failure (HF), the BCS held a dedicated session to review the seminal clinical trials and advances in chronic heart failure management in this period.
Dr Rosita Zakeri (Royal Brompton Hospital, London) reviewed this session for us and spoke to the BJC afterwards.
The era of vasodilator therapy for heart failure began in the 1990s. Professor Karl Swedberg (University of Gothenberg, Sweden) began by outlining a golden period of discovery, marked by V-HeFT-II (Vasodilator-Heart Failure Trial II),2 SOLVD (Studies of Left Ventricular Dysfunction) in 1991,3 and the SAVE (Survival and Ventricular Enlargement) trials4 in 1992, which established renin-angiotensin-aldosterone system (RAAS) inhibitors as the cornerstone of chronic heart failure therapy. Mixed results were obtained for more complete RAAS blockade by a combination of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), and complicated by the higher risk of adverse events (ELITE II,5 CHARM Added,6 ONTARGET7).
The addition of a mineralocorticoid receptor antagonist (MRA), however, demonstrated clear additional reductions in mortality in three trials (RALES,8 EPHESUS9 and EMPHASIS10), confirming the requisite for dual agent RAAS blockade with an ACE inhibitor or ARB and MRA in contemporary chronic heart failure guidelines.11
Glasgow cardiologist, Professor Henry Dargie (University of Glasgow) highlighted the wealth of evidence demonstrating that beta blockers reduce mortality, and sudden death, in patients with chronic heart failure who are in sinus rhythm,12-18 completing the triple therapy regimen (ACE inhibitor or ARB, MRA and beta blocker) for heart failure with reduced ejection fraction (HFREF).11 Importantly, however, recent post hoc and meta-analyses have questioned the role of beta blockers for heart failure patients with atrial fibrillation (AF). The prevailing view is that beta blockers may delay the onset of AF, or may be used to control heart rate. The prognostic benefit for patients with heart failure and AF is unclear.
Device therapy
The evolution of device therapy in heart failure, emerging approximately 10 years after CONSENSUS, was discussed by Professor John Cleland (University of Glasgow). There have been more clinical trials studying device therapy in heart failure than any single pharmacological agent, yielding convincing evidence for cardiac resynchronisation therapy with (CRT-D) or without (CRT-P) an implantable cardioverter defibrillator in younger patients with heart failure (average <70 years) and reduced ejection fraction (HFREF), specifically an ejection fraction below 30%.19-23 On critical examination, there is either weak or insufficient evidence for benefit in patients with an ejection fraction greater than 30%, or in older patients, many of whom receive CRT in real-world practice. The recently published DANISH (Defibrillator Implantation in Patients with Non-Ischaemic Systolic Heart Failure) trial in non-ischaemic HFREF24 calls into question our current selection criteria, and purported benefit of defibrillator therapy in chronic heart failure. Moreover, cost-effectiveness is not achieved until several years after implantation,25,26 which may be a relevant issue for current healthcare systems.
PARADIGM-HF
Finally, Professor John McMurray (University of Glasgow) discussed sacubitril/valsartan, together with the widely reported 20% relative risk reduction in cardiovascular death and heart failure hospitalisation observed in the PARADIGM-HF (Angiotensin-Neprilysin Inhibition versus Enalapril in Heart Failure) trial.27 Recent PARADIGM ancillary studies suggest that patients with low brain natriuretic peptides (BNP) or N-terminal (NT)-proBNP may derive comparable benefit to patients with elevated natriuretic peptides (unpublished data), and additional unexplained effects on glycaemic control may benefit patients with diabetes.28 Verification of these observations in prospective studies is required.
A host of ongoing phase III clinical trials are underway examining sacubitril/valsartan and other agents in chronic heart failure, including the cardiac myosin activator, omecantiv mecarbil (GALACTIC-HF), anticoagulation with rivaroxaban (COMMANDER HF), the soluble guanylate cyclase stimulator, vericiguat (SOCRATES-Reduced, VICTORIA), intravenous iron (IRONMAN, AFFIRM-HF, FAIR-HF2, HEARTFID), and SGLT2 inhibitors dapagliflozin and empagliflozin, as well as the role of influenza vaccination in chronic HF (INVESTED). All the speakers agreed that we still have much work to do, in particular to fill evidence gaps for defining and treating patients with acute heart failure and heart failure with preserved ejection fraction (HFPEF). Nevertheless, the session highlighted the remarkable progress that has been made in defining disease-modifying therapy for chronic HFREF and, with several promising agents under investigation, there is every reason to be optimistic about the future of heart failure care.
Heart failure guidelines – ringing the changes
Expert insights into the recently updated European Society of Cardiology (ESC) guidelines for the diagnosis and treatment of acute and chronic heart failure11 were provided by Professors Frank Ruschitzka (University Heart Centre, Zurich, Switzerland) and John Cleland (University of Glasgow), in a guideline session update at the conference.
Professor Ruschitzka discussed the creation of a new heart failure phenotype, heart failure with mid-range ejection fraction (HFmREF), one of the recent updates to ESC guidance. He emphasised the need for further research into the “middle child” of heart failure given the lack of randomised trials to currently guide our treatment of patients falling within this class.
The inclusion of sacubitril/valsartan in the updated guidelines also provoked much discussion, with the ESC adopting a more conservative recommendation for using this drug compared to the American Heart Association (AHA) and American College of Cardiology (ACC) guidelines. The ESC guidelines recommend using the drug only in HFREF patients, who remain symptomatic despite treatment with beta blockers, angiotensin blockers and MRAs. Professor Ruschitzka stressed that this recommendation was driven by the PARADIGM-HF trial27 and the profile of patients proven to benefit from the drug in the study.
There was also discussion regarding the treatment of patients with ‘left atrial disease’ and the questions that still remain unanswered about the benefit of beta blockade and CRT in HFREF patients and co-existent AF. Other areas requiring further research include the benefit of intervention for functional mitral regurgitation. Professor Ruschitzka highlighted the lack of randomised evidence for the use of percutaneous edge-to-edge repair in this setting.
Professor Cleland gave a personal view on the guidelines in a talk entitled, ‘The good, the bad and the ugly’. He too discussed the inclusion of HFmREF and underlined the importance of using natriuretic peptides to distinguish the condition from other causes of breathlessness. He also pointed out sub-group analyses, which suggest potential benefits with pharmacological therapy in these patients.
The benefit of primary prevention implantable cardioverter defibrillators (ICD) in non-ischaemic cardiomyopathy was also discussed in light of the DANISH trial, which was published after the updated guidelines (see above). Professor Cleland stressed the importance of considering the risks of death from competing causes in decision making, pointing out that patients expected to survive less than five to eight years from implant are unlikely to benefit from an ICD. He also discussed the importance of QRS duration rather than QRS morphology in the selection of patients for CRT and neatly demonstrated that non-ischaemic heart failure may be associated with better outcome following CRT but not better response to the therapy.
Professor Cleland also discussed the need for future studies to focus on end points assessing symptom improvement and quality of life rather than simply mortality. He pointed out the pitfalls in the current classification of evidence used in guidelines which fail to take into account the size and quality of the studies performed and where meta-analyses of small heterogeneous studies can be given a stronger level of evidence than a large, well-performed randomised trial.
Both presentations provided not only an excellent overview of the updated guideline but also an understanding of the challenges encountered when writing them and important areas for future research.
A fresh view on acute heart failure
An indication of the burden of heart failure in the UK was given by Professor Andrew Clark (Castle Hill Hospital, Hull York Medical School, Kingston upon Hull). “Heart failure is now the single most common cause for admission to hospital in people over the age of 65,” he told the meeting. Speaking on acute heart failure at the British Heart Foundation (BHF) ‘Hot topics’ session, he said the big challenge for treating acute heart failure is managing properly patients’ diuretics, as typically, he says, this is done “rather poorly”.
The conventional picture of heart failure, associated with acute pulmonary oedema, “has become quite a rare phenomenon in hospital”, he says. In contrast, much more common is “a sub-acute process” during which, he says, patients retain a lot of fluid (perhaps up to 20 litres or more) over a period of weeks or months.
In an interview with the BJC during the conference, Professor Clark discussed how management of this condition can be optimised.
He also outlined what happens to patients once they have gone home. “We try and offer our patients with an admission for acute heart failure a ‘seamless service’ …and a smooth transition back to primary care”. This crucial transition only works if there is a close relationship between secondary and primary care and that we develop systems whereby GPs will develop an interest in heart failure management. Because it’s so very common, every GP and every nursing practitioner will have to become familiar with the management of patients with heart failure, Professor Clark concluded.
Optimising heart failure treatments: the ‘E’ drugs!
Aware of this need, several sessions at the BCS looked at ‘Cardiology in primary care’ and Professor Theresa McDonagh (Kings College Hospital, London) reviewed treatments for HFREF in one of these sessions entitled ‘What’s new’. We spoke to her during the meeting.
Asking “Is E good for heart failure?”, she highlighted how it is drugs beginning with ‘E’ that are used throughout the heart failure prescribing pathway starting with ACE inhibitors where “the prototype ACE inhibitor, the star of the show”, she says, is enalapril, the drug used in all the original studies in HFREF.
Although ACE inhibitors have brought “huge benefits”, their effect on survival in HFREF is modest, she says, leading to the introduction of other ‘E’ drugs further down the pathway. These include the mineralocorticoid receptor antagonists (MRA), including eplerenone. MRAs have shown a convincing decrease in all-cause mortality in patients with HFREF but there is still a “gap”, she says, with many patients undergoing recurrent hospitalisation.
This has led to the development of “our newest heart failure drug”, sacubitril/valsartan, combining an ARB with a neprilysin inhibitor (ARNI), known by the brand name of EntrestoTM. This has shown a “striking” result in clinical trials, she said, significantly reducing cardiovascular death, hospitalisations and all-cause mortality.
Despite the benefits shown, with the two latter agents in particular, Professor McDonagh points out that “like all heart failure drugs, they are not prescribed as much as we would want them to be”. Despite their clinical trial evidence base and recommendations in guidelines, real-life registry data suggest that only about half of eligible patients are prescribed these drugs. The challenge, she says, is to implement guidelines and make sure our patients get on the best therapy. “These drugs are truly modifying the course of the disease,” she concluded.
Sacubitril/valsartan: initial experience on the front line
Two posters presented at the meeting detailed how both a tertiary centre (Coventry) and large district general hospital (Portsmouth) have successfully implemented services to initiate and monitor sacubitril/valsartan, and the experience that they have had with this agent in a ‘real world’ setting. These posters were moderated by Professor John McMurray (University of Glasgow) and Dr Andrew Ludman (Royal Devon & Exeter NHS Foundation Trust).
Cardiology departments around the UK began to introduce sacubitril/valsartan to patients following the success of the PARADIGM-HF study27 and the subsequent approval by the National Institute for Health and Care Excellence (NICE) of sacubitril/valsartan as a treatment for symptomatic chronic heart failure with reduced ejection fraction (HFREF) in their new technology appraisal TA388.29
The two posters, one from University Hospitals Coventry and Warwickshire Trust and the second from Portsmouth Hospitals NHS Trust, are both discussed in full in an appendix to this report (see Appendix: posters 1 and 2). They give their experience of successfully introducing, monitoring and uptitrating sacubitril/valsartan for HFREF patients using a nurse-led heart failure clinic in Coventry and a registrar-run service in Portsmouth. Although, overall, both centres reported good tolerability of the new agent with many patients reporting a subjective improvement in quality of life, both centres also found some difficulties in assessing the success of introducing sacubitril/valsartan and also that not all patients got on well with the drug. One of the poster presenters, Dr Richard Crawley (Portsmouth Hospitals NHS Trust) also discusses what has been learned from their experiences (see Appendix: discussion posters 1 and 2).
Heart failure management in primary care
Continuing variability of UK heart failure services
Elsewhere at the conference, the challenges for primary care physicians in managing heart failure were discussed. One particular challenge for GPs is the extreme variation across the UK in accessing heart failure diagnostics. This was highlighted by Professor Ahmet Fuat (Durham University and GPSI in cardiology, Darlington) who said that heart failure rates are increasing steadily but there is a question mark on whether “heart failure services actually exist in some areas”.
Speaking after a ‘Cardiology in primary care’ session entitled ‘Which patients should I refer? Which patients should I admit?’, he pointed out that without good access to diagnostics, including BNP tests and echocardiography, the challenge for GPs when they suspect heart failure, is “achieving an accurate diagnosis before we can get a management plan for that patient”.
Professor Fuat said he believed that most heart failure care “can easily be delivered in primary care”. With skilled doctors and nurses, diversification and multi-disciplinary teams, bringing practices together in federations and hubs with general practitioners with special interests (GPSIs), practitioners with special interests (PWSIs), and clinical pharmacists, there is scope to manage patients with long-term conditions.
Speaking to the BJC about the medical management of heart failure, he looks at the increasing spectrum of drugs and their use in primary care where most GPs manage heart failure by following the NICE guidelines,29 he says.
Quality and Outcome Framework (QoF) figures show that GPs are “doing very well in terms of using ACE inhibitors, ARBs and beta blockers”, he says. The use of MRAs is also “gradually increasing”. While the newer agent, sacubitril/valsartan, is mainly specialist prescribed, he believes this can be taken over by GPs. Most areas have a shared protocol where, once the patient is established on the drug, the GP takes over the prescribing. Ivabradine, however, is poorly understood in primary care, he says, and is underused.
Not all breathlessness is heart failure
In the same BCS session, Dr Nigel Rowell (General Practitioner, GPSI in cardiology, Middlesbrough) talked about the common symptom of breathlessness in elderly patients. He spoke to the BJC about a review he undertook of 580 practice attendances with breathlessness over a three-year period, and highlighted how only 28 of these turned out to be heart failure.
What then summates to a high suspicion of heart failure? Dr Rowell advises that this includes elderly patients who experience increasing tiredness, lethargy, depression, or patients who perhaps complain “it’s my age doctor…I can’t get out anymore”. He also highlighted that we should be suspicious of those patients who may not be breathless but may have leg oedema: in particular, men with ankle swelling (which is rare compared to the prevalence in women) may warrant a BNP test, he says.
We should also take note of patients with recurrent chest infections – about 20% of patients with chronic obstructive pulmonary disease will have heart failure. Atrial fibrillation is commonly associated with heart failure and so it is important to feel the pulse, he advises.
“A great gift” to the GP for ruling out heart failure has been the BNP test, he says. If BNP levels are not elevated and a patient’s ECG is ‘normal’, they are unlikely to be suffering from heart failure, he says.
BCS: cardiology trainees have their say
The BCS Annual Conference is a popular meeting for cardiology trainees and results from the 2017 British Junior Cardiologists Association (BJCA) annual survey were presented there, by BHF Clinical Research Training Fellow Dr Abhishek Joshi (Kings College London). He spoke to the BJC about the survey and reported a “record turn out” with almost 500 trainees responding to the survey giving opinions on everything from career choice to bullying during their training.
For the first time, the numbers of trainees aspiring to follow imaging and heart failure careers was greater than for interventional cardiology “which reflects a change in how trainees see the future of cardiology, probably in terms of the job market but also of their levels of interest in those sub-specialties in advanced training modules,” said Dr Joshi. He also discusses the effects of ‘rota gaps’ on access to training in our podcast.
The Young Investigator Award is an annual highlight of the meeting and Dr Chris Allen (St Thomas’ Hospital, London) spoke to us about a common theme of inflammation running through the work of the 2017 award candidates. “Inflammation is the final common pathway for a number of cardiovascular pathologies ranging from post-myocardial infarction to looking at the heart failure population, both with preserved and reduced ejection fraction,” he said.
We also spoke to Dr Brian Halliday (Royal Brompton Hospital, London) at the meeting to find out what’s in the BCS Conference for cardiology trainees who attend.
BJCA President, Dr Jubin Joseph (St Thomas’ Hospital, London), outlined how this years conference theme, ‘Cardiology at the extremes’ provided a perfect backdrop to review all aspects of the discipline from, biomarker definitions of myocardial infarction, to practice in extremes- such as space or in the forefront of military combat, for example.
Appendix: poster 1
Sacubitril/valsartan in chronic symptomatic heart failure with reduced ejection fraction: first clinical experience from a large UK tertiary centre
Danish Ali et al. (University Hospitals Coventry & Warwickshire NHS Trust)
Dr Danish Ali presented a poster based on the work of his team at University Hospital Coventry, a large tertiary cardiac centre in the Midlands, and their assessment of the success of sacubitril/valsartan.
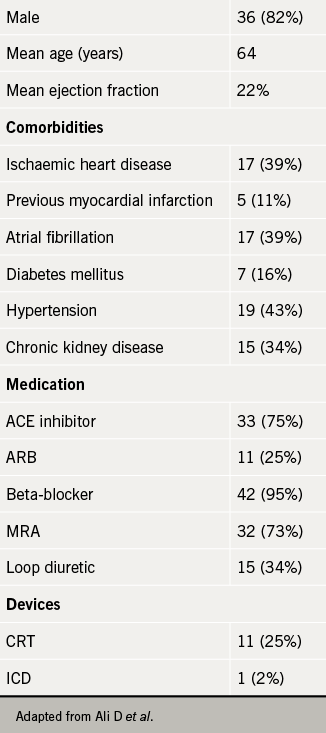
NICE guidance29 explains that sacubitril/valsartan has been recommended for patients with HFREF (i.e. left ventricular ejection fraction (LVEF) ≤ 35%) and should be considered as a treatment for patients with symptoms persistently within New York Heart Association (NYHA) class II to IV, and those who are already taking a stable dose of either ACE inhibitor or an ARB. In most heart failure populations around the UK, this covers a large number of patients, and based on this guidance sacubitril/valsartan could be used as a substitute for either an ACE inhibitor or ARB long-term.
The team retrospectively evaluated 44 patients who were started on a single dose of sacubitril/valsartan (49/51 mg bd) in specialist heart failure clinics between April and October 2016 (see table 1). All patients had either their ACE inhibitor or ARB stopped 48 hours before starting sacubitril/valsartan, and each patient attended a nurse-led heart failure clinic every four weeks until uptitration was complete or deemed not appropriate. Changes in NYHA class, blood pressure readings, and serum potassium and estimated glomerular filtration rate (eGFR) were compared with measurements taken when starting the medication. The team also looked at the proportion of patients achieving the optimal target dose (97/103 mg bd), along with number of hospitalisations and deaths.
Success in uptitration of sacubitril/valsartan was shown by 25 (57%) patients achieving the target dose during the six-month period studied. Twelve (27%) patients showed an improvement in NYHA class, and although moderate improvements in LVEF were not reported, two (5%) patients were found to have an excellent improvement in LVEF from ≤ 35% to ≥ 55%.
Despite this success, not all patients got on with the new medication. Only two of the 44 patients (5%) did not tolerate sacubitril/valsartan and had to stop the medication but nine (20%) patients experienced a symptomatic drop in systolic blood pressure of > 10 mmHg, and three (7%) patients at follow-up had significant hyperkalaemia (K+ > 6.0 mmol/L). Overall, four (9%) patients were admitted to hospital, of which two were due to symptoms of decompensated heart failure, and one was associated with symptoms of hyperkalaemia. Out of those that were admitted, there were two (5%) deaths – one from symptoms of decompensated heart failure and the other of non-cardiovascular cause.
The study overall showed that the real world tolerability of sacubitril/valsartan was very good, and that those who stayed on the medication were likely to improve in terms of both symptoms, and in some cases, LVEF. Those patients in which the target dose was not achieved suffered from both postural hypotension and hyperkalaemia.
Appendix: poster 2
Sacubitril/valsartan: real world experience of delivery and tolerability
Richard Crawley et al. (Portsmouth Hospitals NHS Trust)
This second poster also discussed the real word experience of using sacubitril/valsartan, but from a slightly different angle. This was presented by Dr Richard Crawley who carried out the study alongside the heart failure team at Portsmouth Hospitals NHS Trust.
It was identified early on that converting appropriate patients to sacubitril/valsartan would be a fairly time-intensive process. With only three in-hospital heart failure nurses, who already had both inpatients and outpatients to manage, it was felt that adding uptitration and monitoring of a new and unfamiliar drug would add extra work to an already overburdened nursing team.
“As a first year registrar, I became involved in single-handedly setting up and running clinics to monitor and observe the progress of patients who had been started on sacubitril/valsartan (either 24/26 mg bd or 49/51 mg bd) by the heart failure consultant team,” he writes. During each visit (every two to four weeks), the progress of each patient was documented. “Had they gotten on okay with the drug? Had their energy levels or walking distance improved? Did they have significant light-headedness, or worse, postural hypotension? Any other side effects?”
Blood pressure and heart rate were recorded at each visit, along with the most recent serum biochemical markers (usually creatinine, potassium, eGFR) – these readings would give a greater understanding of the success that patients were having on the new medication. At the end of the consultation, a decision was made as to whether the dose should be increased, and once the patient had reached a stable dose, whether they should be discharged back to their GP.
Over the course of nine months, the team managed to start 120 patients on sacubitril/valsartan, and NICE compliance was reached in all but one patient – a gentleman with extremely symptomatic heart failure who had been trialled on both an ACE inhibitor and ARB, but had not tolerated either (and subsequently did not tolerate sacubitril/valsartan either). In total, only 14 (11.7%) patients did not tolerate the drug and had to revert back to their previous treatment. This is slightly more than the percentage seen in PARADIGM-HF27 (10.7%). Of those that did tolerate the drug, a further eight (7.5%) patients were down titrated to a tolerable lower dose.
Most patients, however, did very well on sacubitril/valsartan, and at time of presentation, 96 (80.0%) of the patients had been discharged back to their GP. The median time needed for follow up was 48 days, with a mean of 2.3 follow up consultation required. Of those discharged, 76 (79.2%) reported a subjective improvement in their quality of life since starting on the drug – mostly with regards to walking distance and overall energy levels. The majority of patients (67; 69.8%) were discharged on the highest dose, 97/103 mg bd– the dose used in the PARADIGM-HF study27 (see figure 1).
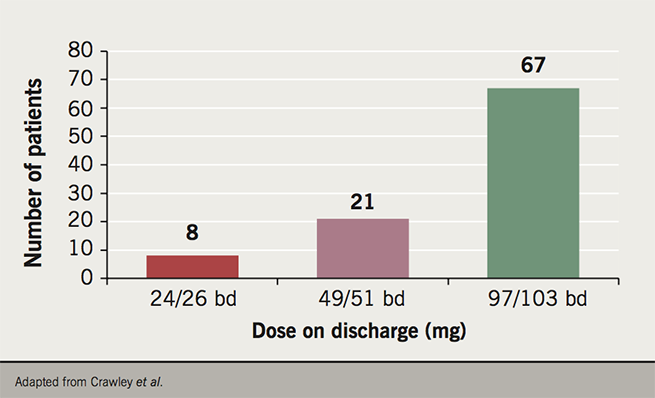
The results were not all positive though. Some 15.0% of patients reported symptomatic hypotension (versus 14.0% in PARADIGM-HF27), and of those, four (3.3%) patients were admitted with syncope as a result. However, during the nine months since the clinics were started, only four patients were admitted with symptoms of decompensated heart failure. Given that the mean LVEF was 27.9% in this group of patients, those numbers are estimated to be slightly lower than expected over the course of that time period. No patients reported angioedema, and although there were slight fluctuations in the serum renal function in some patients, there were no episodes of significant deterioration in kidney functioning after starting sacubitril/valsartan.
Overall, the study reiterated the success in tolerability of the drug in patients with HFREF, and the likelihood of an improvement in quality of life and symptoms. Like the study from Coventry, the vast majority of patients in Portsmouth were able to tolerate the highest dose (97/103 mg bd) without problems. Using a registrar to manage these clinics was also effective, and provided useful experience in the management and psychology of patients with chronic severe heart failure.
Appendix: discussion posters 1 and 2
Richard Crawley
These two fairly similar studies look at the implementation of sacubitril/valsartan in real world patients in two centres in the UK. Both had success in getting patients both onto the drug, and then safely uptitrated. Several questions remain, however, about the types of patients chosen and the overall tolerability of sacubitril/valsartan in the wider heart failure population.
Patients in both studies had a similar mean age (Coventry: 64 years; Portsmouth 62.9 years), which was comparable with the mean age in PARADIGM-HF. However, the average age in most populations suffering from HFREF is higher than that, and closer to 70 years old. Does this mean that both centres were specifically picking patients who they thought would both benefit and tolerate sacubitril/valsartan? What’s more, how were the patients selected? Both presentations implied that those patients selected for initiation onto the new drug were mostly stable outpatients, who were continuing to suffer from significant symptoms. The aetiology of the patients’ heart failure was also poorly touched upon – would patients with ischaemic cardiomyopathy fare better than those with other forms of HFREF? These areas could be considered and addressed in future follow-up studies.
There are, of course, several major studies currently recruiting (e.g. Assessment of Real Life Care – Describing European Heart failure Management – ARIADNE) which will assess the follow up implications, both positive and negative, of sacubitril/valsartan. What both of these posters show, however, is that it is possible to get a significant proportion of the heart failure population onto this novel drug, where there are likely to be significant benefits for patients.
Several issues may still be stopping UK NHS trusts from implementing similar initiatives. Firstly, the cost of the medication is still relatively high, and with the patent due to live for the foreseeable future, the cost-benefit analysis of using sacubitril/valsartan at a local level is likely to be difficult for some trusts. The relatively low level of hospitalisation of those taking the medication in these two studies may be an indication that the expense of the drug compared to ACE inhibitors and ARBs may be counterbalanced by the number of hospital admissions and inpatient days it may help prevent. Further research is needed in the UK before such a conclusion can be reached.
Secondly, the monitoring of new medications is very difficult. Both studies used different methods to monitor and uptitrate the patients taking sacubitril valsartan – Coventry utilised nurse-led heart failure clinics; whilst Portsmouth relied on a supervised registrar to run and lead this monitoring process. Both studies relied on GPs taking over the prescription and monitoring of these patients once stable doses had been reached – agreements which had to be reached with the primary care and pharmacy services locally. It’s unclear from these two observational studies how much work the GP and community heart failure teams may have been doing behind the scenes.
The two posters show that the benefits of initiating sacubitril/valsartan in the HFREF population are starting to emerge. Uptitration and monitoring provide a challenge, however. Perhaps the differing models in these two studies can be utilised by other cardiology teams around the UK, and encourage more heart failure specialists to prescribe sacubitril/valsartan.
References
1. Group CTS. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987;316:1429-35. http://dx.doi.org/10.1056/NEJM198706043162301
2. Cohn JN, Johnson G, Ziesche S et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991;325:303-10. http://dx.doi.org/10.1056/NEJM199108013250502
3. SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB and Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293-302. http://dx.doi.org/10.1056/NEJM199108013250501
4. Pfeffer MA, Braunwald E, Moye LA et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med 1992;327:669-77. http://dx.doi.org/10.1056/NEJM199209033271001
5. Pitt B, Poole-Wilson PA, Segal R et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial- the Losartan Heart Failure Survival Study ELITE II. Lancet 2000;355:1582-7. https://www.ncbi.nlm.nih.gov/pubmed/10821361
6. McMurray JJ, Ostergren J, Swedberg K et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003;362:767-71. http://dx.doi.org/10.1016/S0140-6736(03)14283-3
7. ONTARGET Investigators, Yusuf S, Teo KK et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008;358:1547-59. http://dx.doi.org/10.1056/NEJMoa0801317
8. Pitt B, Zannad F, Remme WJ et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709-17. http://dx.doi.org/10.1056/NEJM199909023411001
9. Pitt B, Remme W, Zannad F et al.Eplerenone Post-Acute Myocardial Infarction Heart Failure E and Survival Study I. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309-21. http://dx.doi.org/10.1056/NEJMoa030207
10. Zannad F, McMurray JJ, Krum H et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11-21. http://dx.doi.org/10.1056/NEJMoa1009492
11. Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. http://dx.doi.org/10.1093/eurheartj/ehw128
12. Packer M, Bristow MR, Cohn JN et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996;334:1349-55. http://dx.doi.org/10.1056/NEJM199605233342101
13. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9-13. http://dx.doi.org/10.1016/S0140-6736(98)11181-9
14. Packer M, Fowler MB, Roecker EB et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 2002;106:2194-9. https://www.ncbi.nlm.nih.gov/pubmed/12390947
15. Flather MD, Shibata MC, Coats AJ et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005;26:215-25. http://dx.doi.org/10.1093/eurheartj/ehi115
16. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001-7. http://dx.doi.org/10.1016/S0140-6736(99)04440-2
17. Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001;357:1385-90. https://www.ncbi.nlm.nih.gov/pubmed/11356434
18. Willenheimer R, van Veldhuisen DJ, Silke B et al. Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence: results of the randomized Cardiac Insufficiency Bisoprolol Study (CIBIS) III. Circulation 2005;112:2426-35. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.582320
19. Bristow MR, Saxon LA, Boehmer J et al. Comparison of Medical Therapy P and Defibrillation in Heart Failure I. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004;350:2140-50. http://dx.doi.org/10.1056/NEJMoa032423
20. Bardy GH, Lee KL, Mark DB et al. Sudden Cardiac Death in Heart Failure Trial I. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225-37. http://dx.doi.org/10.1056/NEJMoa043399
21. Cleland JG, Daubert JC, Erdmann E et al. Cardiac Resynchronization-Heart Failure Study I. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539-49. http://dx.doi.org/10.1056/NEJMoa050496
22. Moss AJ, Hall WJ, Cannom DS et al. Investigators M-CT. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329-38. http://dx.doi.org/10.1056/NEJMoa0906431
23. Tang AS, Wells GA, Talajic M et al. and Resynchronization-Defibrillation for Ambulatory Heart Failure Trial I. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med 2010;363:2385-95. http://dx.doi.org/10.1056/NEJMoa1009540
24. Kober L, Thune JJ, Nielsen JC et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016;375:1221-30. http://dx.doi.org/10.1056/NEJMoa1608029
25. Mark DB, Nelson CL, Anstrom KJ et al. Cost-effectiveness of defibrillator therapy or amiodarone in chronic stable heart failure: results from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Circulation 2006;114:135-42. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.581884
26. Noyes K, Veazie P, Hall WJ et al. Cost-effectiveness of cardiac resynchronization therapy in the MADIT-CRT trial. J Cardiovasc Electrophysiol 2013;24:66-74. http://dx.doi.org/10.1111/j.1540-8167.2012.02413
27. McMurray JJ, Packer M, Desai S, et al. Angiotensin-neprilysin inhibition versus enalapril for heart failure. N Engl J Med 2014;371:993-1004. http://dx.doi.org/10.1056/NEJM0a1409007
28. Seferovic JP, Claggett B, Seidelmann SB et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol 2017;5:333-40. http://dx.doi.org/10.1016/S2213-8587(17)30087-6
29. National Institute for Health and Care Excellence (NICE) Technology appraisal guidance [TA388]. Sacubitril valsartan for treating symptomatic chronic heart failure with reduced ejection fraction. Published 27th April 2016 https://www.nice.org.uk/guidance/ta388
