Background
Table 1 – Typical symptoms and signs of heart failure
| Symptoms | Signs |
|---|---|
| Exertional breathlessness | Laterally displaced apex beat |
| Ankle swelling | Third heart sound |
| Orthopnoea (sensation of breathlessness when lying flat) | Peripheral pitting oedema (commonly ankles or sacrum in bedbound patients) |
| Paroxysmal nocturnal dyspnoea | Raised jugular venous pulse |
| Fatigue | Bi-basal fine crackles |
| Reduced exercise tolerance | Heart murmur |
Heart failure is a clinical syndrome in which patients have typical symptoms and signs due to an abnormality of cardiac structure or function (table 1).1 Its prevalence is high and increasing due to an ageing population, improved survival rates following myocardial infarction, increasing prevalence of risk factors such as diabetes and obesity – and better medical care. Patients with heart failure have a poor quality of life and reduced life expectancy, often more-so than is associated with some cancers. Effective treatment can improve symptoms and outcome but there are wide variations in the management of heart failure across the UK.2
Clinical definition
HeFREF/HeFNEF/HeFMREF
Patients with heart failure are often sub-divided in relation to their left ventricular ejection fraction (LVEF).
- heart failure with a reduced ejection fraction (HeFREF) is due to impaired systolic, contractile function of the left ventricle – but it is important to realise that impaired systolic function does not happen in isolation. Patients with HeFREF invariably also have abnormal diastolic function
- some patients have signs and symptoms of heart failure but have a normal ejection fraction on echocardiography (figure 1). Such patients are variously labelled as having HeFNEF, HeFPEF (where the P stands for ‘preserved’) or ‘diastolic heart failure’ (figure 2).
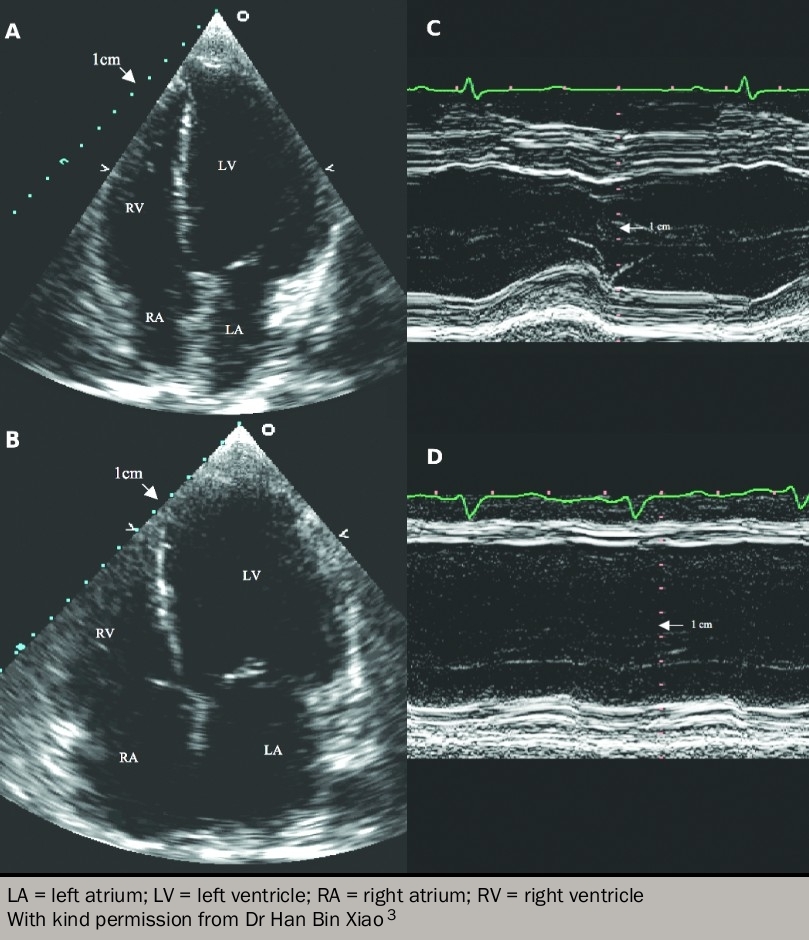
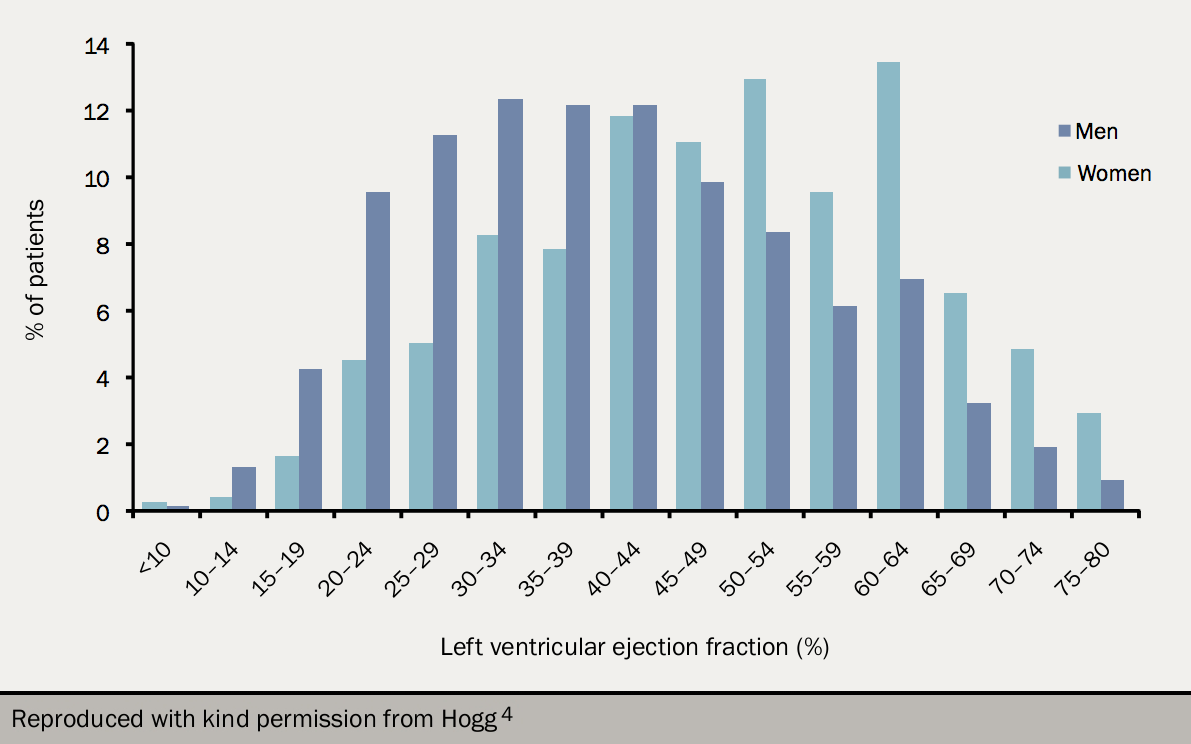
Around half of all patients with heart failure have normal left ventricular systolic function.3,4 When compared to patients with reduced systolic function (HeFREF), those with normal ejection fraction are:5,6
- more likely to be female
- older
- less likely to have coronary artery disease
- more likely to have hypertension
- more likely to have valvular heart disease
- more likely to have atrial fibrillation
- more likely to have diabetes or chronic obstructive pulmonary disease (COPD)
- more likely to have muscle wasting (or sarcopaenia)
- often given different pharmacotherapy
- likely to have lower mortality rates.3
HeFNEF remains a controversial entity, and many believe that there is a significant risk of inappropriate diagnosis.
For example, the definition of a ‘normal’ left ventricular ejection fraction (LVEF) depends upon the method used to measure it and the population studied. Echocardiography is most widely used, but LVEF by echo is poorly reproducible: a patient said to have normal LVEF by one operator may, on a different day, or with a different operator, be said to have reduced ejection fraction.
Additionally, although much quoted epidemiological studies suggest that patients with HeFNEF have much the same prognosis as those with HeFREF,7 every trial population in whom LVEF has been measured reports a linear relation between increasing LVEF and increasing survival.
An additional concern is that whilst ejection fraction may qualify as being ‘normal’ by being above some arbitrary cut-point, systolic function can still be abnormal when measured using other techniques, such as longitudinal strain.
The label applied can also be provocative. Many prefer the term ‘preserved’ to describe a normal ejection fraction – although left ventricular ejection fraction might be in the normal range, it might have fallen to reach that point, and cannot be known to be preserved.
A third heart failure phenotype has been proposed that lies in the ‘grey area’ between HeFREF and HeFNEF: heart failure with mid-range ejection fraction (HeFMREF) with LVEF 40–49%.1
The prevalence of HeFMREF is between 14–18% of patients with heart failure.8–11 Patients with HeFMREF are older and more likely to be female with greater burden of co-morbidities than patients with HeFREF, but with a similar prevalence of ischaemic heart disease (IHD).12 Patients with HeFMREF are thus similar to patients with HeFNEF. However, their prognosis is similar to that of HeFREF and their causes of death are closer to those seen in patients with HeFREF than HeFNEF.13,14
The clinical and laboratory characterstics of patients with HeFMREF are intermediate between HeFNEF and HeFREF.15 It is possible that those with HeFMREF are in transition from HeFNEF to HeFREF or vice versa.16–19 It is not at all clear as yet whether HeFMREF is a useful concept.
Identifying the heart failure phenotype is essential for management
Despite much debate surrounding the value of LVEF as a measure of cardiac function, there is no doubt that it is helpful in defining patients who are likely to respond to specific therapies. Clinical trials have unequivocally demonstrated the benefit of medical and device therapy for patients with heart failure – but only amongst those with reduced ejection fraction, however that is defined. Conversely, there are no interventions proven to improve outcome in patients with HeFNEF.
Table 2. Precipitants and causes of acute heart failure
| Events usually leading to rapid deterioration |
|---|
| Rapid arrhythmia or severe bradycardia/conduction disturbance |
| Acute coronary syndrome |
| Mechanical complication of acute coronary syndrome (e.g. rupture of interventricular septum, mitral valve chordal rupture, right ventricular infarction) |
| Acute pulmonary embolism |
| Hypertensive crisis |
| Cardiac tamponade |
| Aortic dissection |
| Surgery and perioperative problems |
| Peripartum cardiomyopathy |
| Events usually leading to less rapid deterioration |
| Infection (including infective endocarditis) |
| Exacerbation of COPD/asthma |
| Anaemia |
| Kidney dysfunction |
| Non-adherence to diet/drug therapy |
| Iatrogenic causes (e.g. prescription of an NSAID or corticosteroid; drug interactions) |
| Arrhythmias, bradycardia, and conduction disturbances not leading to sudden, severe change in heart rate |
| Uncontrolled hypertension |
| Hypothyroidism or hyperthyroidism |
| Alcohol and drug abuse |
| Key: AHF = acute heart failure; COPD = chronic obstructive pulmonary disease; NSAID = non-steroidal anti-inflammatory drug Reproduced with kind permission from Ponikowski1 |
There is a lack of clarity on how to treat patients with HeFMREF – both National Institute for Health and Care Excellence (NICE) and European Society of Cardiology (ESC) guidelines only recommend treatment with angiotensin converting enzyme (ACE) inhibitors, beta blockers or mineralocorticoid receptor antagonists (MRAs) in patients with an LVEF <40%. However, evidence is emerging that suggests treatment with proven benefit for patients with LVEF <40% may also be beneficial for patients with LVEF 40-49%.20 More work is required.
Acute heart failure versus chronic heart failure
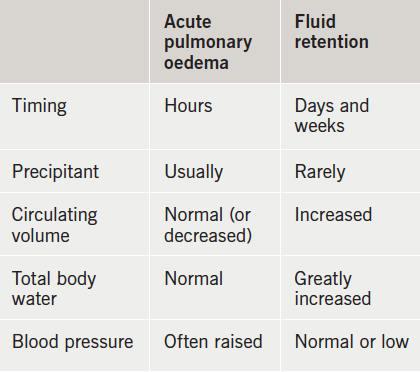
In classifying heart failure syndromes, perhaps the most useful distinction to make is between acute (or decompensated) heart failure and chronic heart failure.
Acute heart failure is the rapid onset, or worsening, of the symptoms and signs of heart failure (table 2). It includes patients with sudden-onset dyspnoea due to acute pulmonary oedema but also those admitted to hospital with fluid retention without breathlessness at rest.
Patients are often thought to lie along a spectrum with acute pulmonary oedema at one end, and gross fluid retention at the other: this may be mistaken; acute pulmonary oedema is a dramatic event precipitated by a discrete pathology such as acute ischaemia or arrhythmia.
An appropriate working definition is that acute heart failure is heart failure that leads to a patient seeking urgent medical attention and often results in hospitalisation whereas chronic heart failure describes the period of stability between any episodes of decompensation.
The pathophysiology of acute heart failure can usually be understood in terms of abnormal haemodynamics, but the pathophysiology of chronic heart failure, especially when treated, is more complex (see later).
Epidemiology
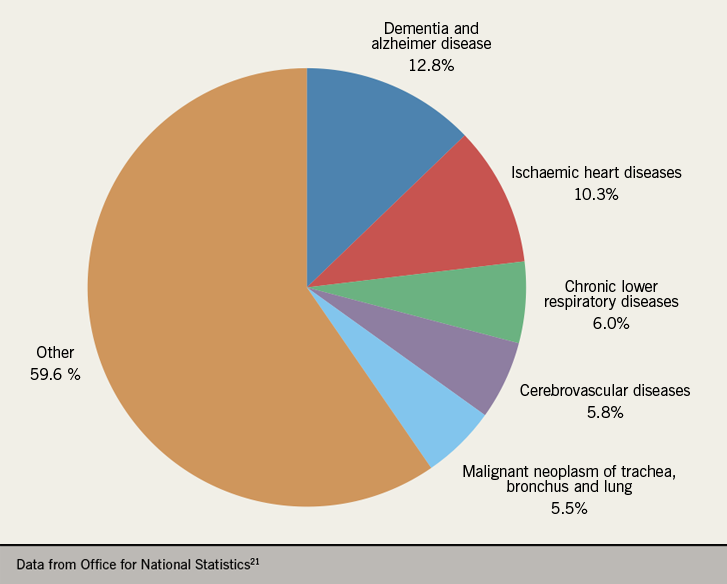
Cardiovascular disease was responsible for one in four deaths in England and Wales during 2018, second only to cancer as the leading cause of death (figure 3).21 Heart failure is the common final end point for most forms of cardiovascular disease; the majority (50–70%) is due to ischaemic heart disease (IHD).3 Recent years have seen a decline in the number of deaths due to IHD and vast improvements in the detection and management of heart failure,22,23,24 resulting in an increase in the number of new cases and the number of patients living with heart failure.25 There are around 920,000 people living with heart failure in the UK – approximately 1.4% of the UK population26 – while many more may be undiagnosed.27
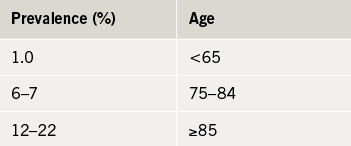
Although age-and-sex standardised prevalence is stable (1–2%), the crude number of patients living with heart failure has increased by 23% in the last decade. Similarly, whilst age and sex standardised incidence of heart failure has decreased in the last decade, the crude number of new cases has increased by 12%. The incidence rises with age and has increased significantly amongst patients aged 85 and older (table 3), probably a result of increased use of screening and diagnostic tests (module 2). The average age at diagnosis is 77 years but is significantly lower in areas of economic deprivation.25
In England and Wales, general practitioners (GPs) keep a heart failure registry as part of the Quality and Outcomes Framework (QOF). These registers report a prevalence of heart failure of only 0.75%,28 suggesting that not all patients with heart failure in primary care are being recorded, or perhaps that the epidemiology is incorrect.
The burden of heart failure
Heart failure has a profound negative impact on the lives of patients and their carers and is costly to manage.
Acute or decompensated heart failure is the leading cause of admission to hospital for patients aged ≥65 years and causes or complicates 5% of all hospital admissions in the UK.2,29 Hospitalisation rates have increased by 33% in the last five years, three times greater than the increases seen for any other condition.30 Re-admission rates are high: as many as 23% will be re-admitted in the month after discharge (although not always for heart failure).31
Mortality is stubbornly high: around 10% of patients admitted with heart failure will die in hospital. Of those who survive admission, approximately 15% will die during the month following discharge and 32% will die in the year following hospital admission.2
Death rates following diagnosis in the community are 19%, 49% and 71% at one, five and 10 years respectively with little appreciable change in the last decade.32
A recent epidemiological study of 56,658 patients registered at general practices in Scotland found that mortality rates for heart failure were similar to that of colorectal cancer and worse than those for bladder, prostate and breast cancer during the 11 year study period from 2000 to 2011 (figure 4).33
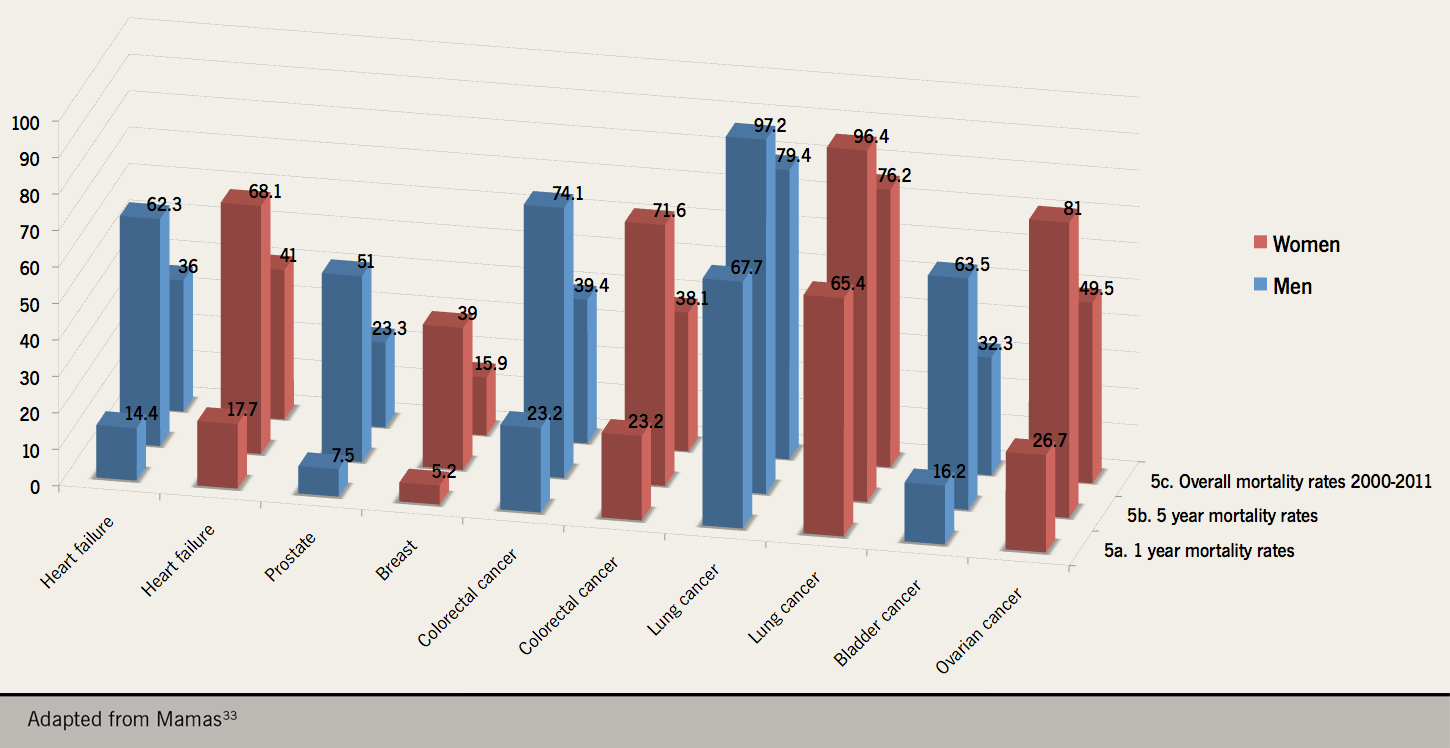
There has been a worrying upward trend in heart failure death rates recently reported in the USA between 2012 and 2017.34 Although comparisons between our state-run health service in the UK and the insurance-based model in the USA are difficult to draw, the increase in hospital admissions with heart failure and the unchanging high death rates during and after admission are concerning.
Admission with heart failure places a huge burden on the patient and health service: it is associated with long hospital stays (~12 days for patients treated on cardiology wards) accounting for 2% of all NHS ‘bed days’. Heart failure accounts for 2% of the entire NHS annual budget (around £625 million per year); 70% is spent on in-patient care and only around 9% is due to drug costs.35
National Heart Failure Audit

The National Heart Failure Audit was established in 2007 and produces annual reports (both as a summary and on a hospital-by-hospital basis) on the demographics, investigations, treatment, place of care, level of specialist input, follow up and outcome of all patients admitted with heart failure in England and Wales.
Records are submitted by individual institutions and, since 2016, have been financially incentivised by the Best Practice Tariff for heart failure. The 2017–18 report captured data from around 76% of all hospital admissions coded as being for heart failure by Hospital Episode Statistics (HES) coding in England and Wales. It is an invaluable source of information on the current ‘state-of-play’ for the quality of care given to patients admitted with heart failure.
Findings from the 2017–18 report
Regarding investigations:
- 86% and 88% of patients received an electrocardiogram (ECG) and echocardiogram (echo) respectively, in order to establish the diagnosis.
- The audit does not record the availability and use of natriuretic peptide measurements, which is probably now the best tool to rule-out heart failure as a cause of presentation. Details of natriuretic peptides can be found in module 2: diagnosis.
- Perhaps unsurprisingly, patients on cardiology wards were more likely to have an echo than patients on general medical wards (95% vs. 84%).
- Patients who had no specialist input were far less likely to have an echo than those who did (69% vs. 92%)
- There has been an approximately 10% drop in the number of patients on general medical wards undergoing echocardiography in the last four years perhaps reflecting a shortage of echo services in secondary care.
- Of those who had an echo; 66% had left ventricular systolic dysfunction, 41% had valve disease, 12% had diastolic dysfunction and 7.3% had left ventricular hypertrophy (LVH).
- These diagnoses are not mutually exclusive.
Regarding specialist input and length of stay:
- 46% of patients are treated on cardiology wards but there is enormous country-wide variation (0-100%); 31% of patients nationally are cared for on general medical wards.
- 82% of patients are seen by a cardiology specialist (consultant cardiologist – 57%, general medical consultant with specialist interest in cardiology or heart failure specialist nurse – 49%).
- Median length of stay is higher for patients on cardiology wards or for those who have received specialist input compared to those in general medicine or who have not seen a specialist (9 days vs. 5-6 days).
- This is possibly a reflection of an increased likelihood of receiving triple-therapy and the time taken to established patients on these medications which, itself, may translate to improved outcomes (see below).
Regarding treatment:
- The majority of patients with left ventricular systolic dysfunction (LVSD) are discharged on either an ACE inhibitor or angiotensin receptor blocker (ARB) (84%) and a beta blocker (89%). Around half of patients with LVSD are discharged on a mineralocorticoid receptor antagonist (MRA).
- 92% are discharged taking a loop diuretic.
- Prescription rates for ACE inhibitors, ARBs, beta blockers and MRAs fall with age, whereas the prescription rate of loop diuretics increases. Overall, prescription rates of all heart failure medications are increasing over time.
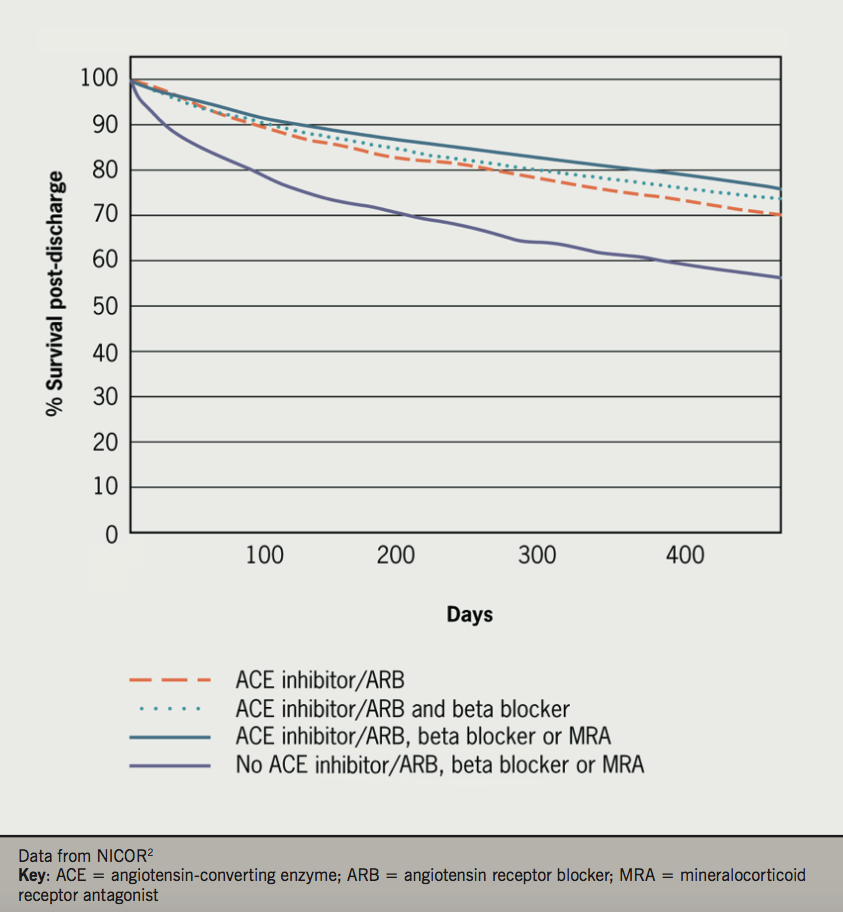
- Patients treated on cardiology wards or under the care of a specialist are approximately twice as likely to be on ‘triple therapy’ (ACE inhibitor or ARB plus beta blocker plus MRA) than those on general medical wards without specialist input (figure 5)
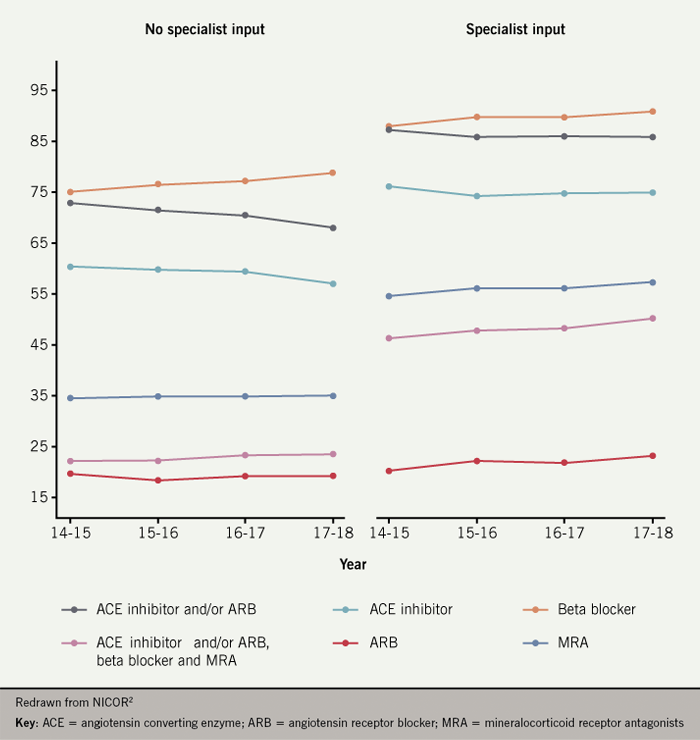
Regarding follow-up and outcome
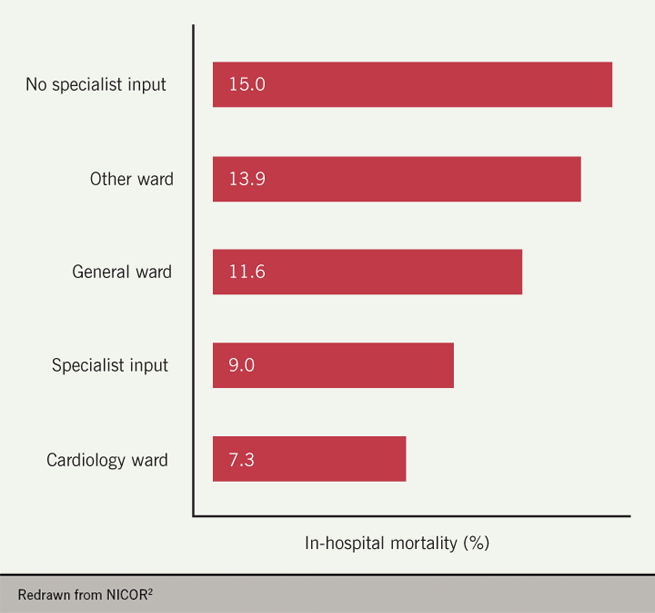
- The inpatient mortality rate for patients who receive no specialist input is 66% higher than patients who receive specialist input (15.0% vs. 9.0%) and more than double that of patients treated on cardiology wards (7.3%) (figure 6).
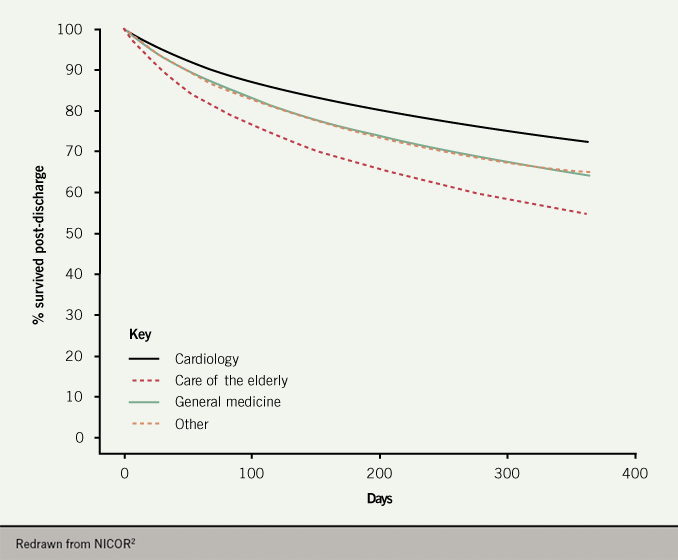
- The beneficial effect of place of care and specialist input as an in-patient persists to one-year post discharge (figure 7).
- Patients who have cardiology follow-up have a lower mortality rate than those who do not (24% vs. 39%).
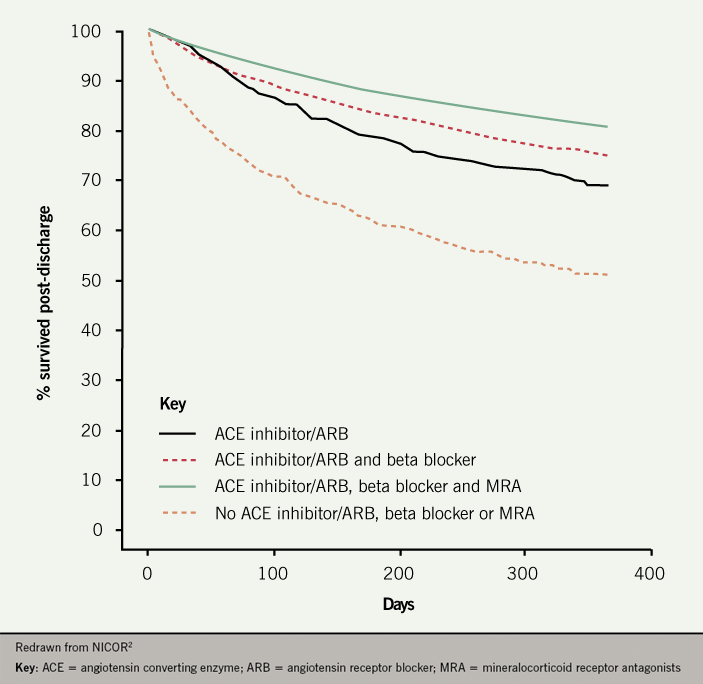
- Post-discharge mortality is highly dependent on treatment: one-year mortality rate for patients receiving ‘triple therapy’ is 19% compared to 48% for patients on no ACE inhibitor, ARB, beta blocker or MRA (figure 8).
QOF indicators, quality standards and best practice tariffs
In the UK, various initiatives are in place to attempt to ensure good quality and even standard of care for patients with acute and chronic heart failure in the in-patient or community setting.
QOF indicators
The performance of general practices in managing patients with heart failure is monitored by the Quality and Outcomes Framework (QOF). QOF, under the guidance of NICE, have developed ‘indicators’ for managing patients with heart failure that act as targets that are financially incentivised (table 4).36
Table 4. QOF indicators related to heart failure, their description and number of QOF points allocated
| QoF indicator | Description | Points allocated |
|---|---|---|
| HF001 | The contractor establishes and maintains a register of patients with heart failure | 4 |
| HF002 | 50–90% of patients with a diagnosis of heart failure (diagnosed on or after 1 April 2006) confirmed by an echocardiogram or by specialist assessment 3 months before or 12 months after entering on to the register | 6 |
| HF003 | 60–100% of patients with a current diagnosis of heart failure due to left ventricular systolic dysfunction treated with an ACE inhibitor or ARB | 10 |
| HF004 | 40–65% of patients with a current diagnosis of heart failure due to left ventricular systolic dysfunction who are currently treated with an ACE inhibitor or ARB and beta blocker | 9 |
| Key: ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; QOF = quality and outcomes framework | ||
The most recent set of QoF indicators (2019–20) no longer rewards practices for referring patients to an exercise-based cardiac rehabilitation programme despite recommendations made in both ESC and NICE chronic heart failure guidelines. This is perhaps a reflection of service-underfunding and the so-called ‘postcode lottery’: exercise-based cardiac rehabilitation can improve symptoms and reduces the risk of heart failure hospitalisation in patients with HeFREF,37,38 but access to such programmes is greatly varied nationwide.
Around 0.7% of patients at general practices nationwide are on their practice’s heart failure register, somewhat below the national heart failure prevalence of 1-2%; the missing patients may be cases of undiagnosed or untreated heart failure.27 Improved coding of electronic records using simple automated methods can greatly increase the apparent prevalence of heart failure,27 and may yield financial reward via the QoF framework.
Quality standards
NICE has produced ‘quality standards’ for acute and chronic heart failure that define the standard of care that should be provided.
Acute heart failure quality standard:39
- Adults presenting to hospital with new suspected acute heart failure have a single measurement of natriuretic peptide.
- Adults admitted to hospital with new suspected acute heart failure and raised natriuretic peptide levels have a transthoracic Doppler 2D echocardiogram within 48 hours of admission.
- Adults admitted to hospital with acute heart failure have input within 24 hours of admission from a dedicated specialist heart failure team.
- Adults with acute heart failure due to LVSD are started on, or continue with, beta blocker treatment during their hospital admission.
- Adults admitted to hospital with acute heart failure and reduced LVEF are offered an ACE inhibitor and an aldosterone antagonist.
- Adults with acute heart failure have a follow up clinical assessment by a member of the community – or hospital-based specialist heart failure team within two weeks of hospital discharge.
Chronic heart failure quality standard:40
- Adults with suspected chronic heart failure who have been referred for diagnosis have an echocardiogram and specialist assessment.
- Adults with suspected chronic heart failure and either a previous myocardial infarction (MI) or very high levels of serum natriuretic peptides, who have been referred for diagnosis, have an echocardiogram and specialist assessment within two weeks.
- Adults with chronic heart failure due to LVSD are started on low dose ACE inhibitor and beta blocker medications that are gradually increased until the target or optimal tolerated doses are reached.
- Adults with chronic heart failure have a review within two weeks of any change in the dose or type of their heart failure medication.
- Adults with stable chronic heart failure have a review of their condition at least every six months.
- Adults with stable chronic heart failure are offered an exercise-based programme of cardiac rehabilitation.
- Adults with chronic heart failure referred to a programme of cardiac rehabilitation are offered sessions during and outside working hours, and the choice of undertaking the programme at home, in the community or in a hospital setting.
Problems with the quality standards
The quality standards require significant resources and do not take into account patient variables that may limit their treatment. They represent a very high standard that many centres may struggle to attain.
- Quality standards for acute heart failure require that:
- All patients to have specialist input within the first 24 hours of admission
- Data from the National Heart Failure Audit shows only 82% of patients get any input from heart failure specialists while an inpatient2
- All patients get a transthoracic echocardiogram within 48 hours of admission
- Only 88% of patients get an echocardiogram at all during admission
- All patients are started or continue on beta blocker during admission and that all patients are offered treatment with ACE inhibitor, ARB and MRA
- Co-morbidities such as renal dysfunction and hypotension are common amongst patients admitted with heart failure and limit the initiation (and, in some cases, require the discontinuation) of heart failure medications. In practice only 89%, 84% and 53% of patients are taking a beta blocker, ACE inhibitor or ARB and MRA respectively
- All patients have follow-up with either their GP, a cardiologist, or heart failure specialist nurse within two weeks of discharge
- This must be one of the most unrealistic targets in the modern NHS.
- All patients to have specialist input within the first 24 hours of admission
Best practice tariff
In 2010 the Department of Health in the UK introduced ‘Best Practice Tariffs’; financial incentives for hospitals to meet what was defined as ‘best practice’ for a particular condition. Base payments for treating a certain condition were reduced overall but additional money was available for meeting criteria based on national guidance and expert opinion that defined ‘best practice’ for managing that condition.41
The best practice tariff for heart failure is worth a 10% increase in payment for every admission, but it is not paid on a patient by patient basis: either the hospital meets the best practice tariff criteria and gets the 10% increase in payment for every admission – or it does not.
The best practice tariff for heart failure was introduced in 2016 and includes two criteria:42
- 60% of patients admitted with failure must receive specialist input during admission as recorded in the national heart failure audit
- 70% of patients coded as having heart failure must be included in the national heart failure audit.
Syndromes and pathophysiology
Over many years, the heart failure syndrome has attracted a number of different terminologies of varying degrees of usefulness. It makes sense, perhaps, to consider the separate heart failure syndromes only where the pathophysiology of them is distinct: the differences highlight important differences in therapeutic approach.
The most useful distinction to make is between acute and chronic heart failure. However, even then, the terms can be confusing. Here, we will take acute heart failure to describe patients with sufficiently severe symptoms and signs that they present acutely seeking medical attention (and are often admitted to hospital); whereas chronic heart failure describes the syndrome patients have once acute heart failure has been medically treated.
Acute heart failure
Most patients (~80%) with heart failure are initially diagnosed during a hospitalisation.43 Acute heart failure is a problem of fluid distribution. Broadly, patients divide into those with acute pulmonary oedema, where there is fluid in the airspaces in the lung, and those with peripheral oedema, where the problem is fluid retention.
Pulmonary oedema
Pulmonary oedema is an acute medical emergency. Patients present with severe breathlessness, which is usually of abrupt onset. The experience for the patient is terrifying: she has to sit upright, is barely able to speak and often fears that she will die.
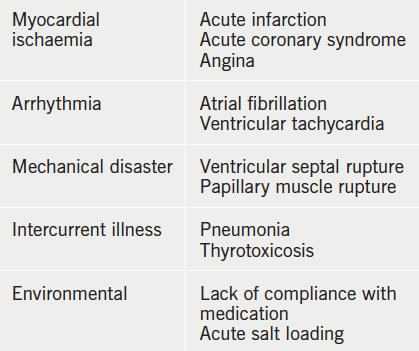
The breathlessness is often accompanied by wheeze and cough productive of pink frothy sputum. There is usually an acute precipitant that has triggered the episode of illness (table 5).
On examination there is massive sympathetic nervous system activation, and the patient is thus usually pale and clammy, with a tachycardia and often hypertension. There is usually a gallop rhythm and widespread crackles and wheezes through the lung fields.
Pathophysiology
Pulmonary oedema is best understood in haemodynamic terms. Fluid is held in the pulmonary capillaries by the balance between the hydrostatic pressure in the capillary tending to push fluid out and the colloid osmotic pressure (largely generated by plasma proteins) tending to hold fluid in the vascular space. There is a net (small) constant transudation of fluid from the pulmonary capillaries which is removed by the lymphatics.
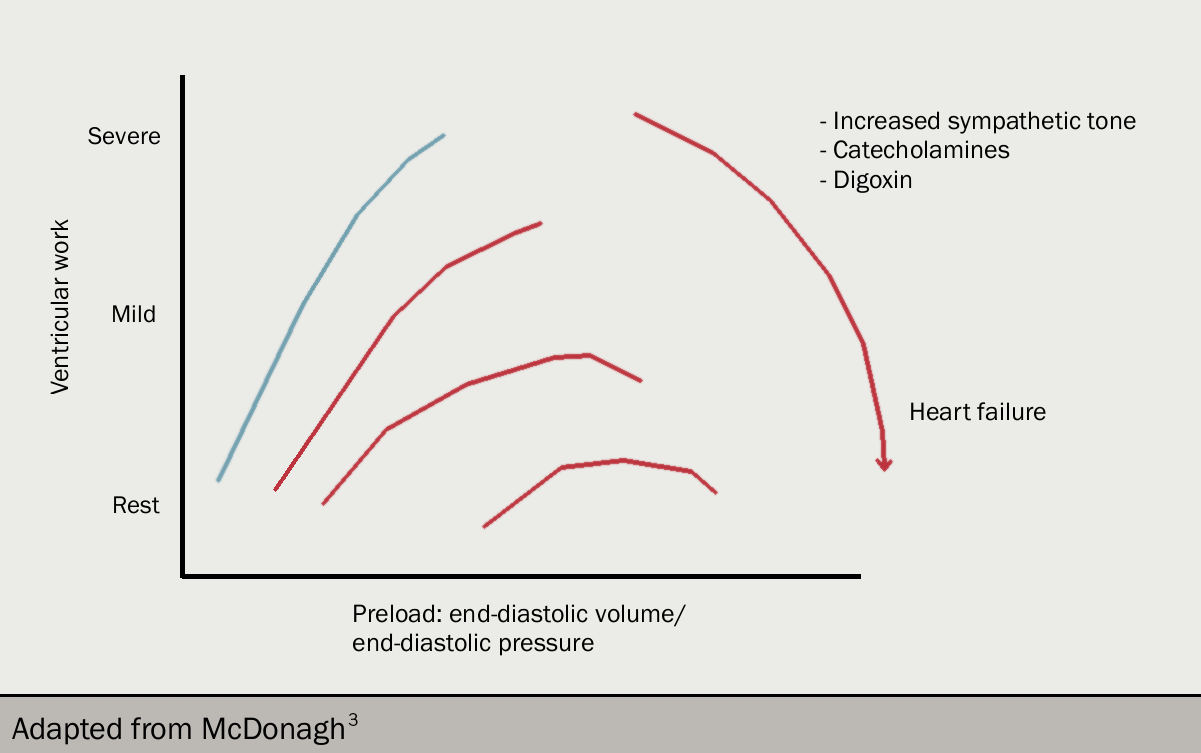
Left ventricular work (and cardiac output) is determined by left ventricular filling pressure (that is, end-diastolic pressure). This is the Frank-Starling relation (figures 10 and 11): as filling pressure rises, so does cardiac output. In the acutely failing left ventricle, the relation is shifted downward and to the left so that to maintain any given cardiac output, a higher filling pressure is required.

As the filling pressure is the same as the pulmonary venous pressure, the rise results in an increase in the rate of transudation from pulmonary capillaries into the lung tissues, and the rate eventually exceeds the rate at which fluid can be removed by the lymphatics. At this point, fluid accumulates in the interstitium of the lung and then the alveoli (figure 12).
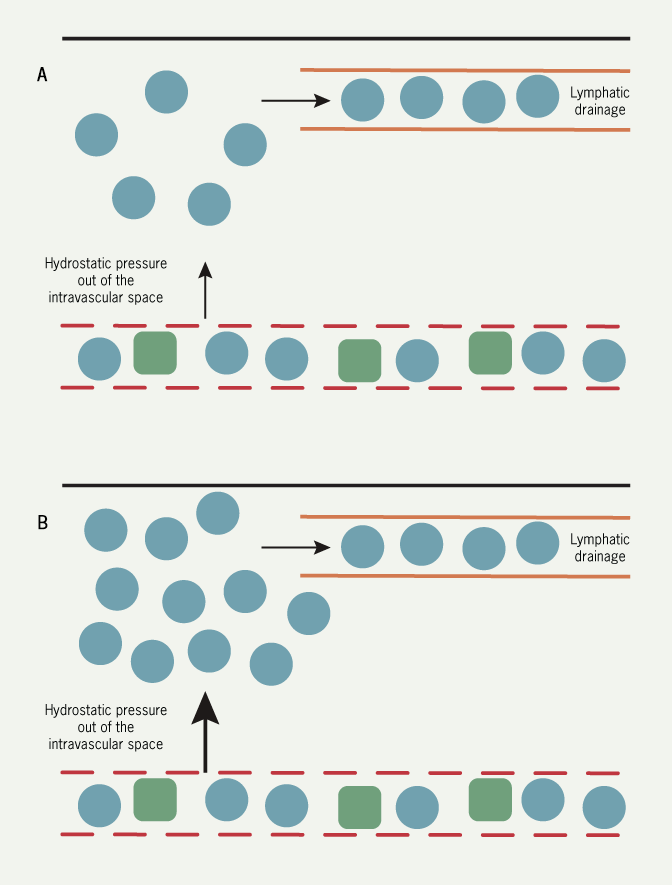
A point often ignored is that the increase in filling pressure requires an input of energy: this can only come from the right ventricle. In acute pulmonary oedema, there must be a temporary imbalance between the output of the two ventricles: the relative excess output from the right results in the increase in left ventricular diastolic pressure and represents the fluid accumulating in the lungs.
Peripheral oedema
More common than pulmonary oedema as a cause of hospitalisation is fluid retention (table 6). In contrast with patients with pulmonary oedema (who may have a low circulating volume due to accumulation of fluid in the lungs), patients with peripheral oedema have an absolute excess of body fluid and an increased circulating volume. The fluid tends to accumulate gradually over many days or weeks; it takes around 5L of excess fluid before peripheral oedema appears.
The order of deposition of oedema is dictated by gravity, so for most patients it starts in the ankles and works upward – and may involve the abdominal, and even thoracic, wall. Patients are usually not breathless at rest, although are unable to do much exercise.
On examination the patient may have a tachycardia with a low volume pulse and a low blood pressure. Around 25% will be in atrial fibrillation. The jugular venous pressure is invariably raised, but may be so high that the top of the column of blood cannot be seen even with the patient sitting upright. Pitting oedema usually starts in the feet and ankles, but be wary of the bed-bound patient in whom sacral oedema may be prominent.
In most patients, the heart is dilated and there is a loud third and often fourth heart sound. Some degree of pulmonary venous hypertension is almost invariable, and patients usually have basal crackles in both lung fields.
Pathophysiology
Peripheral oedema develops in much the same was as pulmonary oedema. The increased circulating volume causes in an increase in hydrostatic pressure (with gravity ensuring that the hydrostatic pressure is highest in the feet). The capacity of the lymphatics to drain away the transudate is exceeded, and oedema fluid starts to form.
What is less certain is why there is salt and water retention in the first place. It may be related to the primary need for the body to maintain blood pressure: if, as a consequence of the failing heart, blood pressure falls, the resultant neurohormonal activation (involving renin-angiotensin-aldosterone system [RAAS], antidiuretic hormone and sympathetic system activation) causes salt and water retention in the kidneys. Additionally, reduced renal perfusion is another stimulus to renin production.
This cannot be the whole explanation as in some patients oedema develops despite normal blood pressure; and in others, there is fluid retention despite aggressive treatment with neurohormonal antagonists.
Chronic heart failure
Although some patients with fluid retention are thought of as having chronic heart failure, particularly if they do not actually need to be hospitalised, the term is best reserved for patients with treated acute heart failure.
Successful treatment of acute heart failure results in patients entering a chronic disease state: they usually have some degree of exercise limitation, but where it is well treated, do not have congestion and oedema.
Chronic heart failure is iatrogenic in the sense that before modern therapies were available, acute heart failure had a very bleak prognosis. Diuretics and treatment with neurohormonal antagonists allows chronic heart failure to develop.
The cardinal symptom of chronic heart failure is exercise limitation. Patients complain of breathlessness or fatigue on exertion, with the dominant symptom varying with the kind of exercise performed. The origin of symptoms is not clear and is likely to be a result of abnormal haemodynamics, neurohormonal activation and skeletal muscle changes.
Pathophysiology
Haemodynamics: In most patients with chronic heart failure, remodelling causes progressive dilation of the left ventricle. Stroke volume is maintained by an increase in left ventricular wall tension by the law of Laplace.
Cardiac output is usually normal at rest and during submaximal exercise, but may not be able to rise normally during more strenuous activity. However, there is little relation between haemodynamic variables, particularly when measured at rest, and exercise capacity.
Neurohormonal activation: The insight that many neurohormonal systems are activated in chronic heart failure has been the key to the success of modern medical therapy for heart failure:
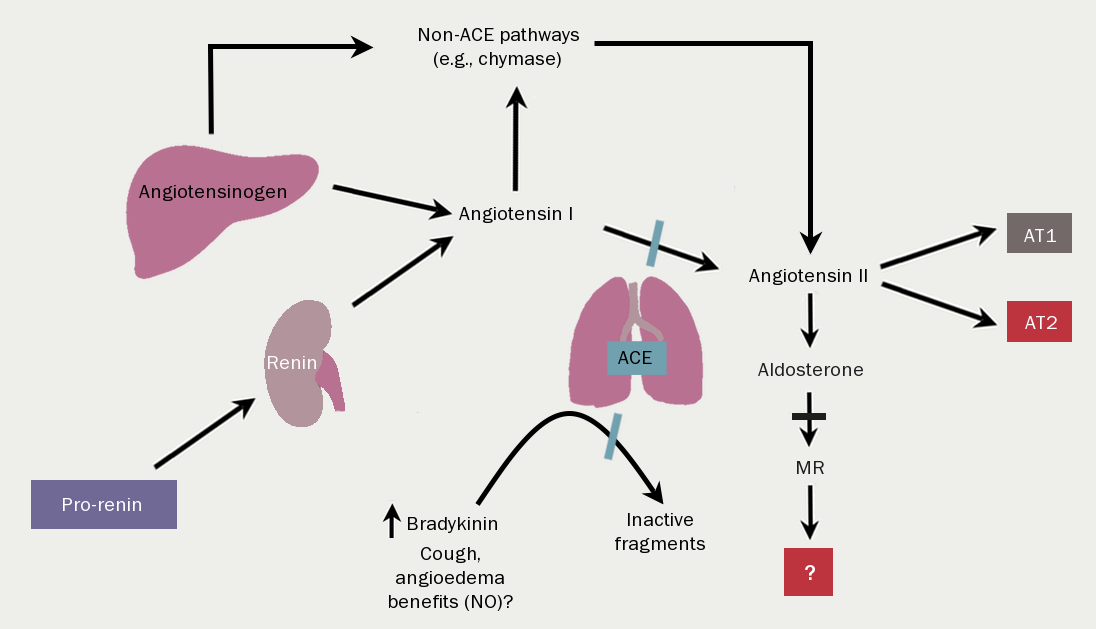
The renin-angiotensin-aldosterone system (RAAS) (figure 13) is activated by reduced renal perfusion. With worsening NYHA class symptoms, so the concentrations of angiotensin and aldosterone rise. Of note, the introduction of diuretic therapy results in further RAAS activation. The consequences are, inter alia, vasoconstriction, salt and water retention, increased vascular fibrosis and a positive interaction with…
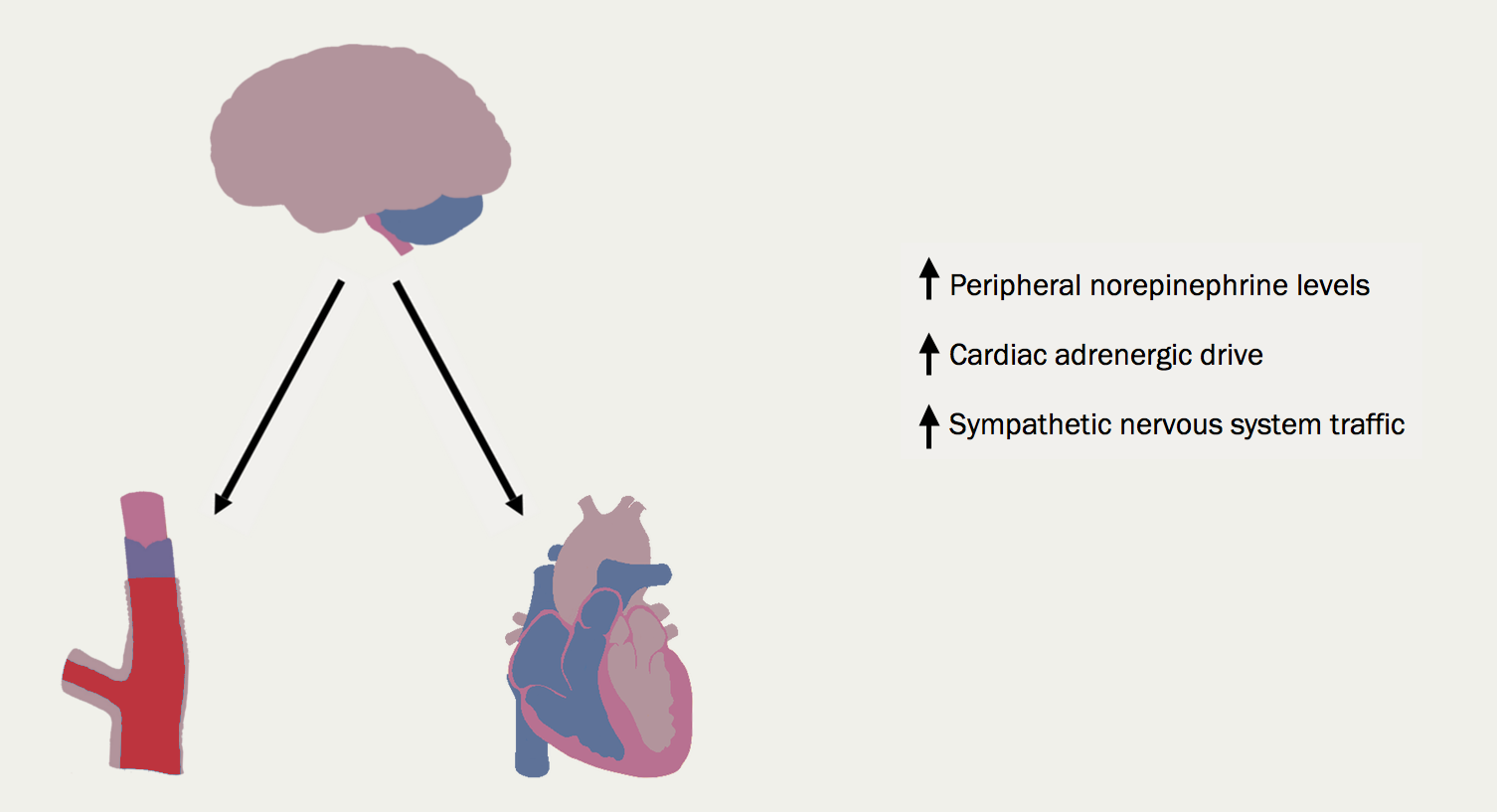
The sympathetic nervous system (figure 14). With worsening heart failure, so sympathetic nervous system activation and concentrations of circulating noradrenaline increase. The consequences for the heart include tachycardia, myocardial fibrosis and apoptosis, increased risk of arrhythmia and peripheral vasoconstriction (figure 15).
Natriuretic peptide concentrations rise as a homeostatic response to dilation of the cardiac chambers. Antidiuretic hormone (arginine vasopressin) is also increased, further enhancing water retention. Other hormone systems are activated, although their clinical relevance is not always clear. The potent vasoconstrictor, endothelin, is raised, and more intriguingly, there is activation of components of the immune system, with increases in tumour necrosis factor and c-reactive protein, for example.
Skeletal muscle changes: these can be very prominent in some patients resulting in cachexia, but almost all patients have some degree of loss of lean muscle bulk. The skeletal muscle changes correlate closely with impaired exercise capacity, and appear to be implicated, at least in part, as the cause of sympathetic nervous system activation.
Peripheral changes: these include the down-regulation of baroreceptors (making them unlikely to be the source of sympathetic nervous system activation) and enhancement of chemoreceptors. The ergoreflexes, a neurally mediated reflex arising from exercising muscle in proportion to work done, is enhanced.
Complications
Arrhythmias are common complications of heart failure, especially atrial fibrillation (AF) (figure 16) which can increase the risk of stroke and thromboembolism or ventricular arrhythmias.1
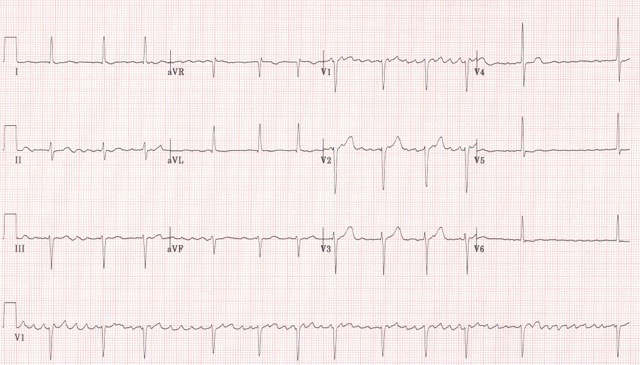
Atrial fibrillation
If patients with heart failure develop AF (figure 16) either permanent or paroxysmal, should be assessed for potentially reversible causes and stroke risk. Unless there is a very strong contra-indication, patients with heart failure and AF should be anticoagulated.
Potential reversible causes of AF in heart failure
- hyperthyroidism
- electrolyte disorders
- uncontrolled hypertension
- mitral valve disease.
Potential precipitating factors of AF in heart failure
- recent surgery
- chest infection or exacerbation of COPD/asthma
- acute myocardial ischaemia
- alcohol binge.
Ventricular arrhythmias
Episodes of asymptomatic, non-sustained ventricular tachycardia are common. “Complex ventricular arrhythmias”, include frequent premature ventricular complexes and non-sustained ventricular tachycardia are associated with a poor outcome.1
Co-morbidities
Patients with heart failure have a wide range of co-morbidities in part related to age. Some co-morbidities may cause heart failure in the first place, for example, cancer after treatment with chemotherapy. Others are risk factors for developing heart failure, such as obesity or hypertension (see below). Polypharmacy is a common consequence and can be very challenging.
Co-morbidities of heart failure include:
• Anxiety and depression
View details
• Anaemia
View details
• Diabetes
View details
• Chronic kidney disease (CKD)
View details
• COPD
View details
• Hyperuricaemia and gout
View details
• Hypertension
View details
• Obesity
View details
• Sleep disturbances
View details
Conclusion
Heart failure has a profound impact on quality of life, prognosis and survival of those affected. Its incidence and prevalence rise with age, and are rising in the population due to better healthcare and therapeutic advances. A large proportion of the health care budget is spent in heart failure hospitalisations.
The pathophysiology of heart failure remains key to understanding how to diagnose and manage the different types of heart failure accordingly.
In following modules, we will explore investigations, diagnosis algorithm and pharmacological and non-pharmacological options for the management of heart failure.
close window and return to take test
References
- Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 2016;18:891–975 http://dx.doi.org/10.1002/ejhf.592
- National Institute for Cardiovascular Outcomes Research. The National Heart Failure Audit Summary Report 2018-2019 (2019). Available from: https://www.nicor.org.uk/wp-content/uploads/2019/09/Heart-Failure-2019-Report-final.pdf [Accessed 15 December 2019]
- McDonagh TA, Gardner RS, Clark AL, Dargie H (ed.). Oxford Textbook of Heart Failure. Oxford: Oxford University Press, July 2011. http://dx.doi.org/10.1093/med/9780199577729.001.0001
- Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol 2004;43:317–27 https://doi.org/10.1016/j.jacc.2003.07.046
- Ferrari R, Böhm M, Cleland JGF, et al. Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail 2015;17:665–71 https://doi.org/10.1002/ejhf.304
- Pellicori P, Cleland JG. Heart failure with preserved ejection fraction. Clin Med (Lond) 2014;14 Suppl 6:s22–8 https://doi.org/10.7861/clinmedicine.14-6-s22
- Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006;355:260–9 https://doi.org/10.1056/NEJMoa051530
- He KL, Burkhoff D, Leng WX et al. Comparison of left ventricular structure and function in Chinese patients with heart failure and ejection fractions >55% versus 40 to 55% versus <40%. Am J Cardiol 2009;103:845–51 https://doi.org/10.1016/j.amjcard.2008.11.050
- Steinberg BA, Zhao X, Heidenreich PA et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction. Prevalence, outcome and therapies. Circulation 2012;126:65–75 https://doi.org/10.1161/CIRCULATIONAHA.111.080770
- Solomon SD, Anavekar N, Skali H et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005;112:3738–44 https://doi.org/10.1161/CIRCULATIONAHA.105.561423
- Fonarow GC, Stough WG, Abraham WT et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure. J Am Coll Cardiol 2007;50:768–77 https://doi.org/10.1016/j.jacc.2007.04.064
- Kapoor JR, Kapoor R, Ju C, et al. Precipitating clinical factors, heart failure characterization, and outcomes in patients hospitalized with heart failure with reduced, borderline, and preserved ejection fraction. J Am Coll Cardiol 2016;4:464–72 https://doi.org/10.1016/j.jchf.2016.02.017
- Gottdiener JS, McClelland RL, Marshall R et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med 2002;137:631–9 https://doi.org/10.7326/0003-4819-137-8-200210150-00006
- Pascual-Figal DA, Ferrero-Gregori A, Gomez-Otero I et al. Mid-range left ventricular ejection fraction: Clinical profile and cause of death in ambulatory patients with chronic heart failure. Int J Cardiol 2017;240:265–70. https://doi.org/10.1016/j.ijcard.2017.03.032
- Rickenbacher P, Kaufmann BA, Maeder MT et al. Heart failure with mid-range ejection fraction: a distinct clinical entity? Insights from the Trial of Intensified versus standard Medical therapy in Elderly patients with Congestive Heart Failure (TIME-CHF). Eur J Heart Fail 2017;19:1586–96 https://doi.org/10.1002/ejhf.798
- Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol 2014;63(25 Pt A):2817–27. https://doi.org/10.1016/j.jacc.2014.03.034
- Clarke CL, Grunwald GK, Allen LA et al. Natural history of left ventricular ejection fraction in patients with heart failure. Circ Cardiovasc Qual Outcomes 2013;6:680–6 https://doi.org/10.1161/CIRCOUTCOMES.111.000045
- Lam CS, Teng TH. Understanding Heart Failure With Mid-Range Ejection Fraction. JACC Heart Fail 2016;4(6):473–6. https://doi.org/10.1016/j.jchf.2016.03.025
- Banerjee P. Heart failure: a story of damage, fatigue and injury? Open Heart. 2017;4(2):e000684. http://dx.doi.org/10.1136/openhrt-2017-000684
- Cleland JGF, Bunting KV, Flather MD, et al. Beta blockers in Heart Failure Collaborative Group. Beta blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J. 2018;39(1):26-35. https://doi.org/10.1093/eurheartj/ehx564
- The Office for National Statistics. Deaths. London: United Kingdom Government, 2019. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathsregisteredinenglandandwalesseriesdrreferencetables [accessed 28 November 2019]
- Smolina K, Wright FL, Rayner M, Goldacre MJ. Determinants of the decline in mortality from acute myocardial infarction in England between 2002 and 2010: linked national database study. BMJ 2012;344:d8059. https://doi.org/10.1136/bmj.d8059
- Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010;362:2155–65. https://doi.org/10.1056/NEJMoa0908610
- Gerber Y, Weston SA, Berardi C, et al. Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: a community study. Am J Epidemiol 2013;178:1272–80. https://doi.org/10.1093/aje/kwt109
- Sutherland K. Bridging the quality gap: heart failure. The Health Foundation. March 2010. Available from http://www.health.org.uk/publications/bridging-the-quality-gap-heart-failure/ [Accessed 28 November 2019]
- Conrad N, Judge A, Tran J, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391(10120):572–80. https://doi.org/10.1016/S0140-6736(17)32520-5
- Cuthbert JJ, Gopal J, Crundall-Goode A, Clark AL. Are there patients missing from community heart failure registers? An audit of clinical practice. Eur J Prev Cardiol 2019;26(3):291–8. https://doi.org/10.1177/2047487318810839
- NHS Digital. Quality and outcomes framework (QOF) – 2018–19. Available from: https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/general-practice-data-hub/quality-outcomes-framework-qof [accessed 10th December 2019]
- National Institute for Health and Care Excellence. Acute heart failure: diagnosis and management. CG187. 2014. Available at: https://www.nice.org.uk/guidance/cg187. [Accessed 11th December 2019]
- British Heart Foundation. Heart Failure hospital admissions rise by a third in five years. Available from: https://www.bhf.org.uk/what-we-do/news-from-the-bhf/news-archive/2019/november/heart-failure-hospital-admissions-rise-by-a-third-in-five-years [Accessed 15th December 2019]
- Martin GP, Kwok CS, Van Spall HGC, et al. Readmission and processes of care across weekend and weekday hospitalisation for acute myocardial infarction, heart failure or stroke: an observational study of the National Readmission Database. BMJ Open. 2019;9(8):e029667. https://doi.org/10.1136/bmjopen-2019-029667
- Taylor CJ, Ryan R, Nichols L, Gale N, Hobbs FR, Marshall T. Survival following a diagnosis of heart failure in primary care. Fam Pract. 2017;34(2):161–8. https://doi.org/10.1093/fampra/cmw145
- Mamas MA, Sperrin M, Watson MC, et al. Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland. Eur J Heart Fail 2017;19:1095–104 https://doi.org/10.1002/ejhf.822
- Sidney S, Go AS, Jaffe MG, Solomon MD, Ambrosy AP, Rana JS. Association Between Aging of the US Population and Heart Disease Mortality From 2011 to 2017. JAMA Cardiol 2019;4(12):1280–6 https://doi.org/10.1001/jamacardio.2019.4187
- Sutherland K. The Health Foundation. Bridging the quality gap: Heart failure. Available from: https://www.health.org.uk/sites/default/files/BridgingTheQualityGapHeartFailure_0.pdf [Accessed 1st December 2019]
- UK Government. Summary of QoF Indicators 2019-20. Available from: https://www.england.nhs.uk/wp-content/uploads/2019/05/gms-contract-qof-guidance-april-2019.pdf [Accessed 9th December 2019]
- Taylor RS, Sagar VA, Davies EJ, et al. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev 2014;4:CD003331. https://doi.org/10.1002/14651858.CD003331.pub4
- O’Connor CM, Whellan DJ, Lee KL, et al. and the HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1439–50. https://doi.org/10.1001/jama.2009.454
- National Institute for Health and Clinical Excellence QS103. Acute heart failure quality in adults. London: NICE, 2016. Available from https://www.nice.org.uk/guidance/qs103/chapter/List-of-quality-statements [Accessed 10th December 2019]
- National Institute for Health and Clinical Excellence QS103. Chronic heart failure in adults. London: NICE, 2016. Available from https://www.nice.org.uk/guidance/qs9/chapter/List-of-quality-statements [Accessed 10th December 2019]
- Department of Health. Payment by results guidance for 2010–11. Leeds: Payment by results team. Available from: http://webarchive.nationalarchives.gov.uk/20130105041537/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/@ps/documents/digitalasset/dh_112970.pdf [Accessed 10th December 2019]
- NHS England. 2017/18 and 2018/19 National Tariff Payment System:a consultation notice Annex B6: Guidance on best practice tariffs. Available from: https://improvement.nhs.uk/documents/484/Annex_DtD_Best_practice_tariffs.pdf [Accessed on 15th December 2019]
- Bottle A, Kim D, Aylin P, Cowie MR, Majeed A, Hayhoe B. Routes to diagnosis of heart failure: observational study using linked data in England. Heart 2018;104(7):600–5. https://doi.org/10.1136/heartjnl-2017-312183
- Celano CM, Villegas AC, Albanese AM, Gaggin HK, Huffman JC. Depression and Anxiety in Heart Failure: A Review. Harv Rev Psychiatry 2018;26(4):175–84. https://doi.org/10.1097/HRP.0000000000000162
- Groenveld HF, Januzzi JL, Damman K, et al. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol 2008;52(10):818-27. https://doi.org/10.1016/j.jacc.2008.04.061
- Westenbrink BD, Visser FW, Voors AA, et al. Anaemia in chronic heart failure is not only related to impaired renal perfusion and blunted erythropoietin production, but to fluid retention as well. Eur Heart J 2007;28:166–71. https://doi.org/10.1093/eurheartj/ehl419
- Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur. Heart J 2013;34(11):816–29. https://doi.org/10.1093/eurheartj/ehs224
- Cleland JG, Zhang J, Pellicori P, et al. Prevalence and Outcomes of Anemia and Hematinic Deficiencies in Patients With Chronic Heart Failure. JAMA Cardiol 2016;1(5):539-47. https://doi.org/10.1001/jamacardio.2016.1161
- Lehrke M, Marx N. Diabetes Mellitus and Heart Failure. Am J Med 2017;130(6S):S40-S50. https://doi.org/10.1016/j.amjmed.2017.04.010
- Voors AA, van der Horst IC. Diabetes: a driver for heart failure. Heart 2011;97(9):774–80. https://doi.org/10.1136/hrt.2009.183624
- de Silva R, Rigby AS, Witte KK, et al. Anemia, renal dysfunction, and their interaction in patients with chronic heart failure. Am J Cardiol 2006;98(3):391-8. https://doi.org/10.1016/j.amjcard.2006.01.107
- de Silva R, Nikitin NP, Witte KK, et al. Incidence of renal dysfunction over 6 months in patients with chronic heart failure due to left ventricular systolic dysfunction: contributing factors and relationship to prognosis. Eur Heart J 2006;27(5):569–81. https://doi.org/10.1093/eurheartj/ehi696
- Hillege HL, Nitsch D, Pfeffer MA, et al. with the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Investigators. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 2006;113(5):671–8. https://doi.org/10.1161/CIRCULATIONAHA.105.580506
- van Deursen VM, Urso R, Laroche C, et al. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail. 2014;16(1):103-11. https://doi.org/10.1002/ejhf.30
- Cuthbert JJ, Kearsley JW, Kazmi S, et al. The impact of heart failure and chronic obstructive pulmonary disease on mortality in patients presenting with breathlessness. Clin Res Cardiol. 2019;108(2):185–93. https://doi.org/10.1007/s00392-018-1342-z
- Spieker LE, Ruschitzka FT, Lüscher TF, Noll G. The management of hyperuricemia and gout in patients with heart failure. Eur J Heart Fail. 2002;4(4):403-10. https://doi.org/10.1016/S1388-9842(02)00086-7
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288(23):2981–97 https://doi.org/10.1001/jama.288.23.2981
- He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med 2001;161(7):996-1002. https://doi.org/10.1001/archinte.161.7.996


All rights reserved. No part of this programme may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publishers, Medinews (Cardiology) Limited.
It shall not, by way of trade or otherwise, be lent, re-sold, hired or otherwise circulated without the publisher’s prior consent.
Medical knowledge is constantly changing. As new information becomes available, changes in treatment, procedures, equipment and the use of drugs becomes necessary. The editors/authors/contributors and the publishers have taken care to ensure that the information given in this text is accurate and up to date. Readers are strongly advised to confirm that the information, especially with regard to drug usage, complies with the latest legislation and standards of practice.
Healthcare professionals should consult up-to-date Prescribing Information and the full Summary of Product Characteristics available from the manufacturers before prescribing any product. Medinews (Cardiology) Limited cannot accept responsibility for any errors in prescribing which may occur.