Exercise training is associated with positive health outcomes in people with cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM). However, fear of hypoglycaemia is a potential barrier to participants attending a cardiac exercise class. Therefore, we assessed the capillary blood glucose (CBG) responses to the Imperial NHS Trust cardiac exercise class.
Forty patients (median age 66 years, interquartile range [IQR] 57–74 years) with CVD and T2DM treated with insulin and/or sulfonylureas completed a cardiac exercise class. CBG was measured immediately before and after the exercise class. Subgroup analysis assessed CBG levels in patients who had consumed food <2 and ≥2 hours and had taken their insulin and/or sulfonylureas <4 and ≥4 hours before the exercise class.
Overall, post-exercise CBG had significantly decreased (–3.0 mmol/L, p≤0.0001). Subgroup analyses demonstrated significant reductions in CBG in both food consumption groups (<2 hours –2.9 mmol/L, p≤0.0001, and ≥2 hours –3.1 mmol/L, p≤0.0001) and medication groups (<4 hours –3.4 mmol/L, p≤0.0002, and ≥4 hours –2.7 mmol/L, p≤0.0001). However, there were no significant differences in CBG between the food consumption groups and the medication groups, respectively (p=0.7 and p=0.3).
Cardiac exercise classes resulted in significant reductions in CBG levels. However, the timing of food consumption or medication intake did not influence the magnitude of CBG decline after the cardiac exercise class.
Introduction
It has been estimated that around one in four people who attend a cardiovascular prevention and rehabilitation programme (CPRP) have diabetes mellitus (DM) and many more are at risk of this condition.1 CPRPs provide an ideal opportunity to help support people with DM through optimisation of their medical therapies, diet and exercise. However, medications such as insulin and insulin secretagogues (sulfonylureas) increase the risk of hypoglycaemia during aerobic exercise.2,3 Repeated bouts of hypoglycaemia increase the risk of cardiovascular mortality,4,5 hypoglycaemia unawareness,6 disability,7 and reduce physical activity adherence in people with DM.8
In order to lower the risk of hypoglycaemic episodes, established guidelines recommend measuring capillary blood glucose (CBG) before and after structured aerobic exercise in people who have been prescribed insulin and/or sulfonylureas.3 However, to date there are no published data that have investigated the need to measure CBG responses to a CPRP exercise class in people with established cardiovascular disease (CVD) and DM. Therefore, the purpose of this service evaluation was to investigate pre- and post-CBG responses in participants with CVD and type 2 diabetes mellitus (T2DM) who attended the Imperial College NHS Trust Cardiac Health exercise classes. The secondary analysis investigated external factors known to effect exercise-induced changes in CBG.
Method and materials
We collected a single CBG measurement using a validated handheld glucometer9 immediately before and after the Imperial College NHS trust cardiac exercise class in 40 consecutive participants with T2DM who had been prescribed insulin and/or a sulfonylurea. Due to small numbers (n=1) we did not include participants with type 1 diabetes mellitus. The cardiac exercise class included a 15-minute warm-up, 24 minutes of circuit interval training, comprised of aerobic and low-intensity resistance exercises, and a 10-minute cool-down. Participants with self-reported mobility limitations attended a separate low-level exercise class that consisted of a 15-minute warm-up, 15 minutes of chair-based or standing/supported exercise, and a 10-minute cool-down. During the training programme, participants were encouraged to exercise at an intensity range of between 40% and 70% of their age-predicted heart rate reserve maximum and/or a Borg rating of perceived exertion of 11–14.10
Prior to exercise, a pre-class check-in took place, which involved recording the participant’s self-reported timing of their most recent food, fluid and anti-hyperglycaemic medication intake. This information was used to stratify the participants into the following groups: food intake <2 hours and ≥2 hours and medication intake <4 hours and ≥4 hours.
Table 1. Patient demographics
| Demographic | Summary |
|---|---|
| Median age, years (IQR) | 66 (57–74) |
| Ethnicity, % South Asian | 70% |
| Gender, % male | 75% |
| Median body mass index, kg/m2 (IQR) |
27.2 (25–31) |
| Waist, cm (SD) | 103.7 (15.7) |
| Median HbA1c, mmol/mol (IQR) | 60 (52–79) |
| Clinical history, N (%) | |
| Angina | 22 (55%) |
| Heart failure | 3 (8%) |
| STEMI/NSTEMI | 14 (35%) |
| Valve disease | 1 (2%) |
| Diabetes medications, N (%) | |
| Rapid-acting insulin | 6 (15%) |
| Short-acting insulin | 2 (5%) |
| Long-acting insulin | 8 (20%) |
| Biphasic insulin | 9 (23%) |
| Sulfonylureas | 25 (63%) |
| DPP-4 inhibitors | 13 (32%) |
| GLP-1 receptor agonist | 1 (2%) |
| Metformin | 32 (80%) |
| SGLT-2 inhibitors | 5 (13%) |
| Key: DPP-4 = dipeptidyl peptidase-4; GLP-1 = glucagon-like peptide 1; IQR = interquartile range; NSTEMI = non-ST-elevation myocardial infarction; SD = standard deviation; SGLT-2 = Sodium-glucose cotransporter 2; STEMI = ST-elevation myocardial infarction | |
All data were assessed for parametric assumptions prior to statistical analysis using STATA version 13.0. Parametric data were expressed as mean ± standard deviation (SD) and non-parametric data as median ± interquartile range (IQR). Paired samples t-tests were conducted on the changes in the pre- and post-exercise class CBG. Unpaired sample t-tests were conducted to compare the differences between groups. Confidence intervals (CI) for the differences between means were calculated and all significance tests were performed at the 5% level.
Results
Data were collected between November 2018 and June 2019. The median age of the patients was 66 years (IQR 57–74 years), 26 (65%) participants took part in the main exercise circuit and 14 (35%) participants took part in the seated/standing supported exercise circuit (table 1).
Overall, CBG significantly decreased from 12.4 ± 3.5 mmol/L pre-exercise to 9.4 ± 3.9 mmol/L post-exercise (–3.0 mmol/L; 95%CI –2.2, –3.7; p≤0.0001). There were no significant differences in the reduction in CBG between the two exercise class modalities (p=0.6).
The changes in CBG in the different food and medication groups are presented in figure 1. In those who had consumed food <2 hours pre-exercise class (n=19), CBG decreased from 12.7 ± 3.6 mmol/L to 9.8 ± 4.0 mmol/L post-exercise class (–2.9 mmol/L; 95%CI –1.6, –4.1; p≤0.0001). In those who had consumed food ≥2 hours pre-exercise class (n=21), CBG decreased from 12.1 ± 3.5 mmol/L to 9.0 ± 3.9 mmol/L post-exercise class (–3.1 mmol/L; 95%CI –2.2, –4.0; p≤0.0001). The reductions in CBG between the two food consumption groups were not significantly different (p=0.7).
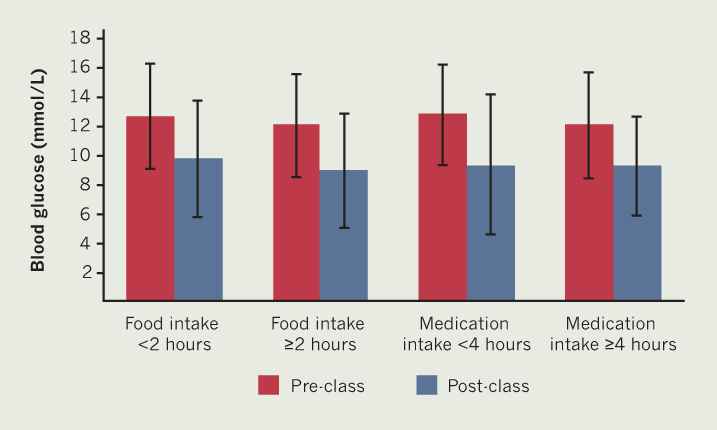
In those who had taken their insulin/sulfonylureas <4 hours before the exercise class (n=21), CBG decreased from 12.8 ± 3.4 mmol/L to 9.4 ± 4.8 mmol/L after the exercise class (–3.2 mmol/L; 95%CI –2.2, –4.2; p≤0.0002). In those who had taken their insulin/sulfonylureas ≥4 hours before starting the exercise class (n=19), CBG decreased from 12.1 ± 3.6 mmol/L to 9.3 ± 3.4 mmol/L post-exercise class (–2.7 mmol/L; 95%CI –1.6, –3.8; p≤0.0001). The reduction in CBG between the two medication timing groups was non-significant (p=0.3).
Discussion
We found that a standard cardiac exercise class11 significantly reduced CBG by 24% post-exercise in those who had been prescribed insulin and/or sulfonylureas. The magnitude of the CBG-lowering effect of a cardiac exercise class and anti-hyperglycaemic medication was highly heterogeneous. For example, one participant experienced a 7.1 mmol/L reduction in their CBG following the exercise class; whereas another person experienced a 2.3 mmol/L increase in their CBG post-exercise class. In addition, one participant experienced a hypoglycaemic episode post-exercise class (pre-CBG 10.8 mmol/L and post-CBG 3.7 mmol/L) despite omitting their morning sulfonylurea dose. This participant was successfully treated with oral glucose gel.
The timing of food and medication intake did not significantly affect the magnitude of the reduction in post-exercise class CBG. Participants consuming food <2 hours and ≥2 hours before the exercise class experienced similar reductions in their post-exercise CBG. However, the timing of insulin and/or sulfonylurea had the most prominent effect in lowering CBG following exercise. Although not significant, there was a trend that participants who had taken their insulin and/or sulfonylurea <4 hours before starting the cardiac exercise class experienced the greatest reductions in their CBG, when compared with those who had taken their medication ≥4 hours (–3.2 mmol/L vs. –2.7 mmol/L). These differences could have been significant with a greater sample size.
There were no significant differences in the reductions in CBG between the two modes of exercise class. However, it is important to note that the seated/standing supported exercise class was shorter in exercise duration compared with the main class. Therefore, a greater reduction in CBG could have been observed in those participating in the low-level exercise class if it was matched to the same duration as the main exercise class. One explanation for these similarities is that participants attending the seated/standing supported class had significant mobility limitations and the majority used a walking aid to ambulate. People with mobility limitations have higher energy costs during daily-living activities and the oxygen cost of walking is twice that of adults with no self-reported physical limitations.12 As a result of higher energy costs the utilisation of CBG into the working muscles will be greater when exercising and during daily-living activities.13
Although the majority of CBG values fell within the normal limits following the cardiac exercise class, the magnitude of the reduction in CBG was highly variable. Therefore, extra care should be taken in monitoring for signs and symptoms of hypoglycaemia and measuring pre- and post-CBG in CVD participants with T2DM who have been prescribed insulin and/or insulin secretagogues. Future research is warranted to assess the CBG response in regular intervals several hours after the exercise session in order to assess post-exercise hypoglycaemia.
Key messages
- This is the first report to analyse the glycaemic responses in people with established cardiovascular disease and type 2 diabetes mellitus who attended a cardiac exercise class. The primary findings indicate that a cardiac exercise class reduces capillary blood glucose (CBG) by 24%
- The timing of food consumption did not appear to affect the magnitude in the reduction of CBG post-exercise. Patients consuming food less than 2 hours and more than 2 hours before starting the exercise class experienced similar reductions in their CBG post exercise
- Similar to the food consumption groups, the timing of anti-hyperglycaemic medication intake did not significantly influence the extent of the reduction of CBG post-exercise. Patients who had taken their anti-hyperglycaemic medication less than 4 hours and more than 4 hours before starting the cardiac exercise class experienced similar reductions in their CBG post-exercise
Conflicts of interest
None declared.
Funding
This service evaluation was funded by the Imperial NHS Cardiac Health and Rehabilitation Service.
Study approval
All procedures, analysis and publication of this report were reviewed and agreed by the Imperial College NHS Healthcare Trust clinical governance board.
Acknowledgement
The authors would like to acknowledge the support of the Imperial College London NHS Healthcare Cardiac Health and Rehabilitation team.
References
1. British Heart Foundation. National Audit of Cardiac Rehabilitation (NACR) Quality and Outcomes Report 2018. London: BHF, 2018. Available from: https://www.bhf.org.uk/informationsupport/publications/statistics/national-audit-of-cardiac-rehabilitation-quality-and-outcomes-report-2018
2. DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med 1999;131:281–303. https://doi.org/10.7326/0003-4819-131-4-199908170-00008
3. Lopez-Jimenez F, Kramer V, Masters B et al. Recommendations for managing patients with diabetes mellitus in cardiopulmonary rehabilitation. An American association of cardiovascular and pulmonary rehabilitation statement. J Cardiopulmonary Rehabil 2011;32:101–12. https://doi.org/10.1097/HCR.0b013e31823be0bc
4. Gerstein HC, Miller ME, Byington RP et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–59. https://doi.org/10.1056/NEJMoa0802743
5. Zoungas S, Patel A, Chalmers J et al. Severe hypoglycaemia and risks of vascular events and death. N Engl J Med 2010;363:1410–18. https://doi.org/10.1056/NEJMoa1003795
6. Graveling AJ, Frier BM. Impaired awareness of hypoglycaemia: a review. Diabetes Metab 2010;36:S64–S74. https://doi.org/10.1016/S1262-3636(10)70470-5
7. Lopez JMS, Annunziata K, Bailey RA, Rupnow MFT, Morisky DE. Impact of hypoglycemia on patients with type 2 diabetes mellitus and their quality of life, work productivity and medication adherence. Patient Prefer Adherence 2014;8:683–92. https://doi.org/10.2147/PPA.S58813
8. Brazeau AS, Rabasa-Lhoret R, Strychar I et al. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care 2008;31:2108–09. https://doi.org/10.2337/dc08-0720
9. Brazg R, Hughes K, Martin P et al. Clinical evaluation of the FreeStyle Precision Pro system. Clin Chim Acta 2013;421:243–50. https://doi.org/10.1016/j.cca.2013.03.021
10. Borg G. Borg’s Perceived Exertion and Pain Scales. Leeds: Human Kinetics, 2008. https://doi.org/10.1037/e529832013-001
11. Association of Chartered Physiotherapists in Cardiovascular Rehabilitation (ACPICR). Standards for physical activity and exercise in the cardiac population. 2015. Available from: https://www.acpicr.com/publications/healthcare-professionals/
12. Malatesta D, Simar D, Dauvilliers Y et al. Energy cost of walking and gait instability in healthy 65-and 80 yr-olds. J Appl Physiol 2003;95:2248–56. https://doi.org/10.1152/japplphysiol.01106.2002
13. Waters R, Mulroy S. The energy expenditure of normal and pathologic gait. Gait Posture 1999;9:207–31. https://doi.org/10.1016/S0966-6362(99)00009-0
