Background
Heart failure (HF) is a clinical syndrome of typical symptoms and signs (figure 1), which occur due to an abnormality of cardiac structure or function, combined with elevated serum natriuretic peptide concentrations and/or objective evidence of pulmonary or systemic congestion on examination.1,2
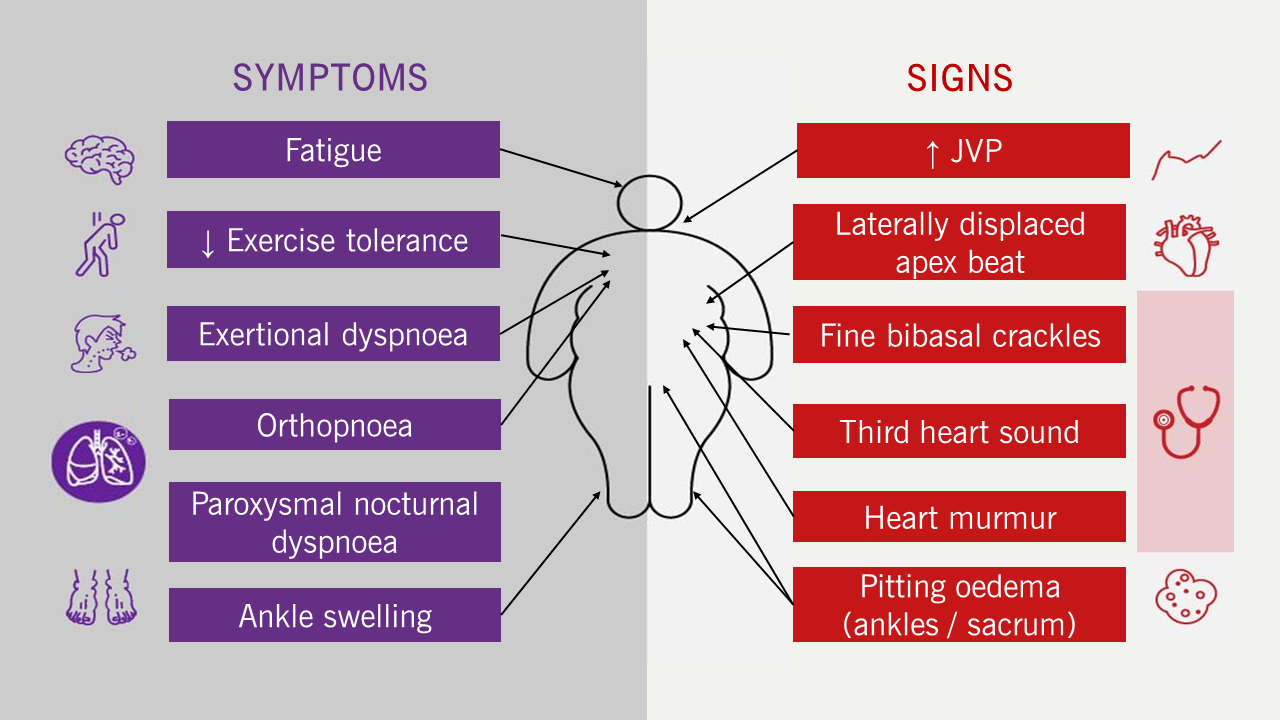
| Key: JVP = jugular venous pressure |
Although everyone learns the mantra ‘HF is a syndrome, not a diagnosis’, HF is most commonly given as a specific diagnosis. This can be problematic as there are multiple possible causes of the syndrome of HF, many of which overlap, and only some of which are due to underlying (and treatable) cardiac dysfunction.
Patients with HF are classified into phenotypes based on the left ventricular ejection fraction (LVEF), most commonly measured using echocardiography. A LVEF ≤40% is classified as HF with reduced ejection fraction (HFrEF); LVEF 41–49% as HF with a mildly reduced ejection fraction (HFmrEF); and LVEF ≥50% as HF with a preserved ejection fraction (HFpEF) (further details will be discussed in module 2).
Epidemiology
Prevalence
The estimated global prevalence of HF is estimated to be 64 million people.2 In the UK, it is estimated that there are more than one million people with HF. HF affects between 1–2% of the general population, which is increasing at a rate of 200,000 new diagnoses every year,3 due to an ageing population, improved survival following myocardial infarction, increasing prevalence of risk factors such as diabetes and obesity – and better medical care. Although age- and sex-standardised prevalence is stable (1–2%), the crude number of patients living with HF has increased by 23% in the last decade.3
Incidence
In a population-based study from the UK including more than four million people, a decline of 7% in the incidence of HF was observed between 2002 and 2014 from 3.6 to 3.3 per 1,000 person-years.2 Whilst age- and sex-standardised incidence of HF has decreased in the last decade, the crude number of new cases has increased by 12%.3
|
National Heart Failure Audit 2024 Report
The recent National Institute for Cardiovascular Outcomes Research (NICOR) National Heart Failure Audit reports that the average age of HF patients in 2022/23 was 77.7 years (75.8 for men and 80.0 for women) with men being more numerous in every age category apart from over 85 years.5 |
The incidence of HF rises with age and has increased significantly amongst patients aged 85 years and older (figure 2), probably as a result of the increased use of screening and diagnostic tests (covered in module 2). The average age at diagnosis is approximately 77 years but is significantly lower in areas of economic deprivation.4 Typical patients are 15–20 years older than the average age of patients recruited to clinical trials.
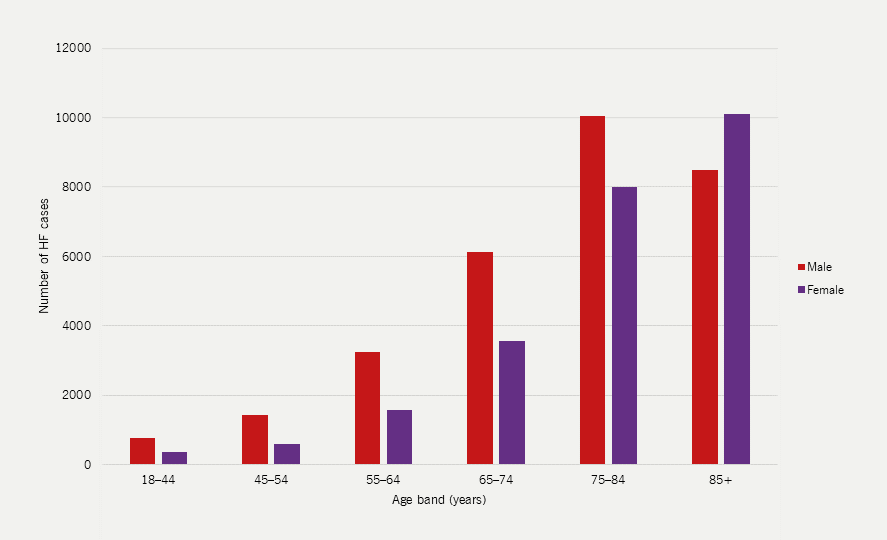
| Adapted with permission from NICOR’s National Heart Failure Audit 2024 Summary Report5 Key: HF = heart failure; NHFA = National Heart Failure Audit |
Outcomes
Untreated, patients with HF have a poor quality of life and reduced life expectancy, more so than with some cancers.6 Most patients are diagnosed only after deteriorating to the point of requiring hospital admission during which approximately one in 10 will die.7 Of those who survive admission, approximately 15% will die during the month following discharge and 32% will die in the year following hospital admission.7 Effective treatment can improve symptoms and outcomes, but there are wide variations in the management of HF across the globe.2
Ischaemic heart disease (IHD) was responsible for one in 10 deaths in England and Wales in 2022, second behind dementia and Alzheimer’s disease as the leading cause of death (figure 3).8 HF is the common final end point for most forms of cardiovascular disease; most (50–70%) are due to IHD.9 Over the last two decades, researchers have seen a fall in the number of deaths due to IHD and huge improvements in the detection and management of HF,4,10,11 resulting in an increased incidence and prevalence of HF.12
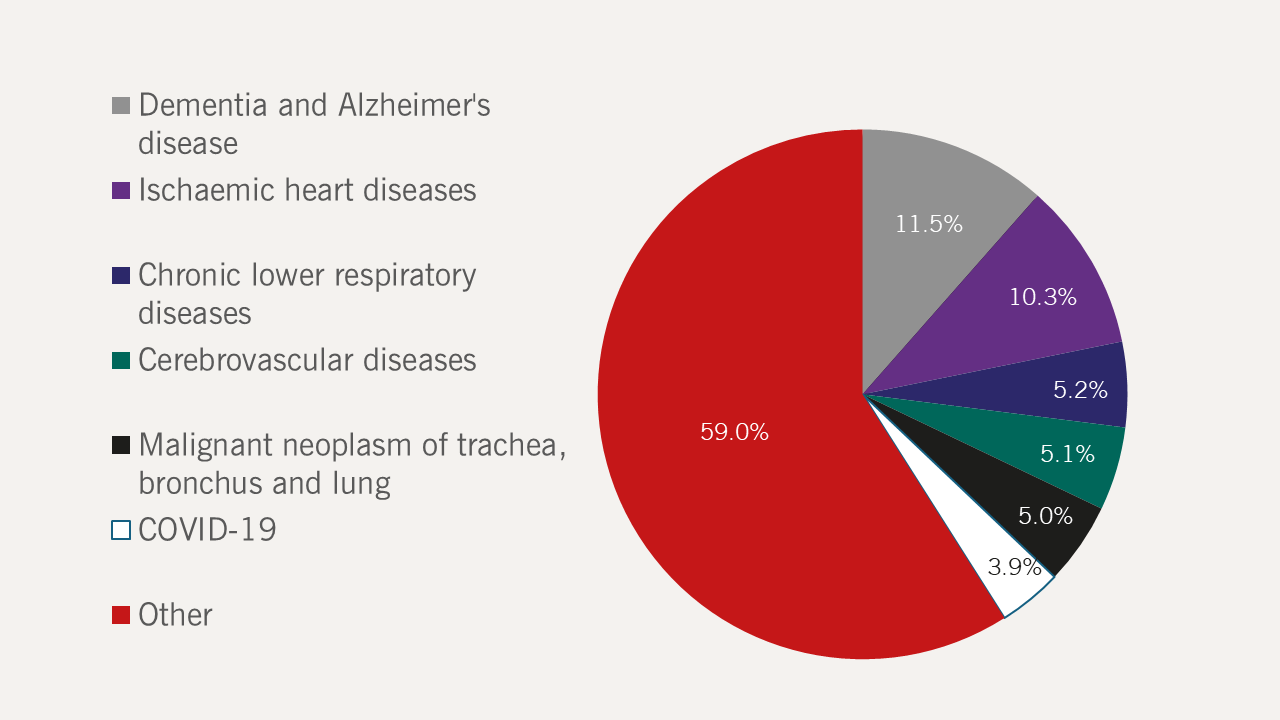
| Data obtained from the Office of National Statistics, 20238 Key: COVID-19 = coronavirus disease 2019 |
QOF indicators
The performance of general practices in managing patients with HF is monitored by the Quality and Outcomes Framework (QOF). Under the guidance of the National Institute for Health and Care Excellence (NICE), the QOF has developed ‘indicators’ for managing patients with HF that act as targets that are financially incentivised (table 1).
In England and Wales, general practitioners (GPs) keep a HF registry as part of the QOF. These registers record a prevalence of HF of only 0.9%,13 suggesting that not all patients with HF in primary care are being recorded, or perhaps that the epidemiology is incorrect.14 Missing patients may be cases of undiagnosed or untreated HF.15 Improved coding of electronic records using simple automated methods can greatly increase the apparent prevalence of HF,15 and may yield financial rewards via the QOF framework.
Table 1. QOF indicators related to heart failure, their description and number of QOF points allocated14
| QOF indicator | Description | Points allocated |
| HF001 | The contractor establishes and maintains a register of patients with heart failure | 4 |
| HF003 | 60–92% of patients with a current diagnosis of heart failure due to left ventricular systolic dysfunction treated with an ACE inhibitor or ARB | 6 |
| HF005 | 50–90% of patients with a diagnosis of heart failure (diagnosed on or after 1 April 2006) confirmed by an echocardiogram or by specialist assessment 3 months before or 6 months after entering on to the register
If newly registered in the preceding 12 months, with no record of the diagnosis originally being confirmed by echocardiogram or specialist assessment, a record of an echocardiogram or a specialist assessment within 6 months of the date of registration |
6 |
| HF006 | 60–92% of patients with a current diagnosis of heart failure due to left ventricular systolic dysfunction, who are currently treated with a beta blocker licensed for heart failure | 9 |
| HF007 | 50–90% of patients with a diagnosis of heart failure on the register, who have had a review in the preceding 12 months, including an assessment of functional capacity and a review of medication to ensure medicines optimisation at maximal tolerated doses. | 7 |
| Key: ACE = angiotensin-converting enzyme; ARB = angiotensin II receptor blocker; QOF = Quality and Outcomes Framework | ||
The National Heart Failure Audit Report 2024: the burden of HF in the UK5

The National Heart Failure Audit was established in 2007 and produces annual reports (both as a summary and on a hospital-by-hospital basis) on the demographics, investigations, treatment, place of care, level of specialist input, follow up and outcome of all patients admitted with HF in England and Wales.
Records are submitted by individual institutions and, since 2016, have been financially incentivised by the Best Practice Tariff for HF (See Best Practice Tariffs for HF). The 2022/23 report captured data from around 86% of all hospital admissions coded as being for HF by Hospital Episode Statistics (HES/PEDW) coding in England and Wales. It is an invaluable source of information on the current ‘state-of-play’ for the quality of care given to patients admitted with HF.
|
Best Practice Tariffs for HF In 2010, the Department of Health in the UK introduced ‘best practice tariffs’. Base payments for treating a certain condition were reduced overall, but additional money was available for hospitals meeting criteria based on national guidance and expert opinion that defined ‘best practice’ for managing that condition.19 The best practice tariff for HF is worth a 10% increase in payment for every admission, but it is not paid on a patient-by-patient basis: either the hospital meets the best practice tariff criteria and gets the 10% increase in payment for every admission coded as HF – or it does not. The best practice tariff for HF, first introduced in 2016, includes two criteria21:
|
HF has a profound negative impact and is costly to manage
Acute or decompensated HF is the leading cause of admission to hospital for patients aged ≥65 and causes or complicates 5% of all hospital admissions in the UK.5,16 Hospitalisation rates have increased by 33% in the last five years, three times greater than the increases seen for any other condition.17 Re-admission rates are high; as many as 23% will be re-admitted in the month after discharge (although not always for HF).18
Mortality is stubbornly high
There was an increase in the number of HF patients admitted to hospital who died to 11% in 2022/23 (from 9% in 2021/22) but 30-day and one-year mortality decreased to 13% and 30%, respectively, down from 14% and 33%, respectively, in 2021/22.5 Perhaps, surprisingly, non-cardiovascular hospitalisation or death accounts for the majority of admissions and deaths in patients with HF,20 which might reflect improvement in HF care, or the fact that HF predominantly affects older people with multiple competing risks.
Death rates following diagnosis in the community are 19%, 49% and 71% at one, five and 10 years, respectively, with little appreciable change in the last decade.22
HF mortality and hospitalisation rates are increasing worldwide
There has been a worrying upward trend in HF death rates recently reported in the USA between 2012 and 2017.23 Although drawing comparisons between our state-run health service in the UK and the insurance-based model in the USA is difficult, the increase in hospital admissions with HF and the unchanging high death rates during and after admission are concerning.
HF creates a huge burden on patients and healthcare systems
Admission is associated with long hospital stays (approximately nine days for patients treated on cardiology wards).5 HF accounts for 2% of the entire National Health Service (NHS) annual budget (around £625 million per year); 70% is spent on in-patient care and only about 9% on drugs.12
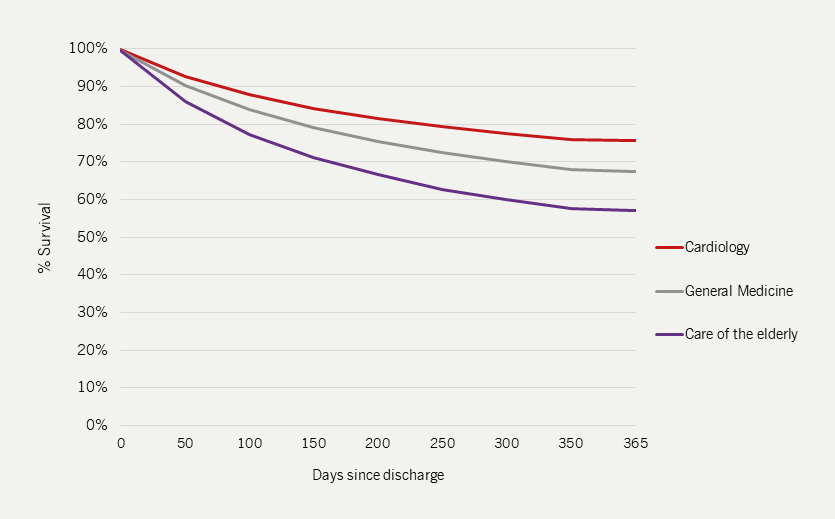
Specialist input improves HF outcomes
|
In-patient mortality is lower for those admitted to cardiology wards and for those who receive specialist care (see figure 4). |
Data from 2022/23 show that:
- Overall 30-day mortality improved to 13% compared to 14% during the 2021/22 audit
- The 60% prescribing target of the three standard outcome-improving drugs for HFrEF (i.e. ACE inhibitors/angiotensin receptor blockers [ARB]/angiotensin receptor-neprilysin inhibitors [ARNI] + mineralocorticoid receptor antagonists [MRA] + beta blocker) was achieved by 54% of hospitals compared to 42% of hospitals in 2021/22. Patients cared for in cardiology wards or seen by specialist teams were more likely to receive outcome-improving drug therapy, including sodium-glucose co-transporter-2 (SGLT2) inhibitors.
- Specialist follow-up within two weeks of discharge was received by 81% of HF patients, similar to 2021/22, but remains short of the 100% target.
A wide variation exists in the level of specialist input
The proportion of patients being admitted to cardiology wards is falling and only 40% of HF patients were cared for on a cardiology ward in 2022/23. Only 15% of hospitals reached the target of 60% of patients being admitted to a cardiology ward and almost 40% of hospitals failed to reach the target of 80% of all HF patients being seen by a specialist HF team. There was wide regional variation with more than a three-fold difference in HF admissions across different health boards in England and Wales.5
Length of stay affected by level of specialist input
Median length of stay was higher for patients on cardiology wards compared to those treated on general medical wards (9 days vs. 7 days).
Likelihood of undergoing all necessary investigations increased with level of specialist input
Patients undergoing echocardiography remained at 89-92% for those receiving specialist care or for those cared for on cardiology wards but this decreased to 65% for those patients not receiving this care.5 The audit does not record the availability and use of natriuretic peptide measurements, which is probably now the best tool to rule-out HF as a cause of presentation. Details of natriuretic peptides can be found in module 2.
Of those who had an echocardiogram, 51% had left ventricular systolic dysfunction, with it being less commonly found in women (40%) than men (59%).5
Prescription of guideline-directed therapy correlates to place of care and level of specialist input
In 2022/23, more patients received drug therapy that improves longer-term outcomes5:
- Beta blockers were prescribed to 91% of patients
- An ACE inhibitor, ARB or ARNI were prescribed to 85% of patients
- MRAs were prescribed to 68% of patients
- SGLT2 inhibitors were prescribed to 59% of patients
- Only 44% of patients received all four classes of outcome-improving drug therapy
- There was considerable variation in prescribing these drugs in different ward settings with highest prescribing rates seen on cardiology wards (figure 5) and with specialist heart failure teams (see figure 6)
- Fewer older patients received disease-modifying drug treatments.
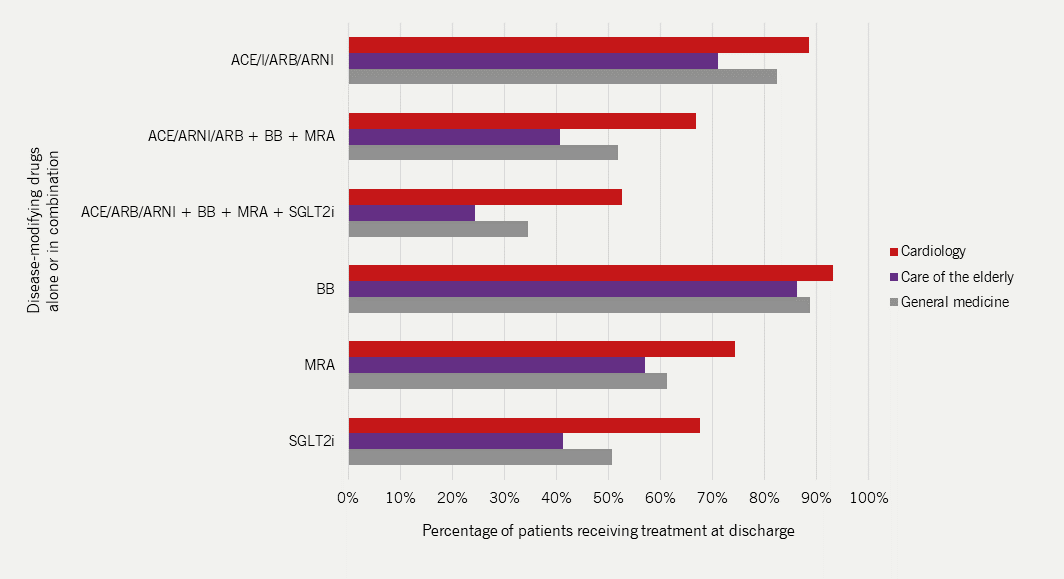
| Adapted with permission from NICOR’s National Heart Failure Audit 2024 Summary Report5 Key: ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; ARNI = angiotensin receptor-neprilysin inhibitor; BB = beta blocker; MRA = mineralocorticoid receptor antagonist; SGLT2i = sodium-glucose co-transporter-2 inhibitor |
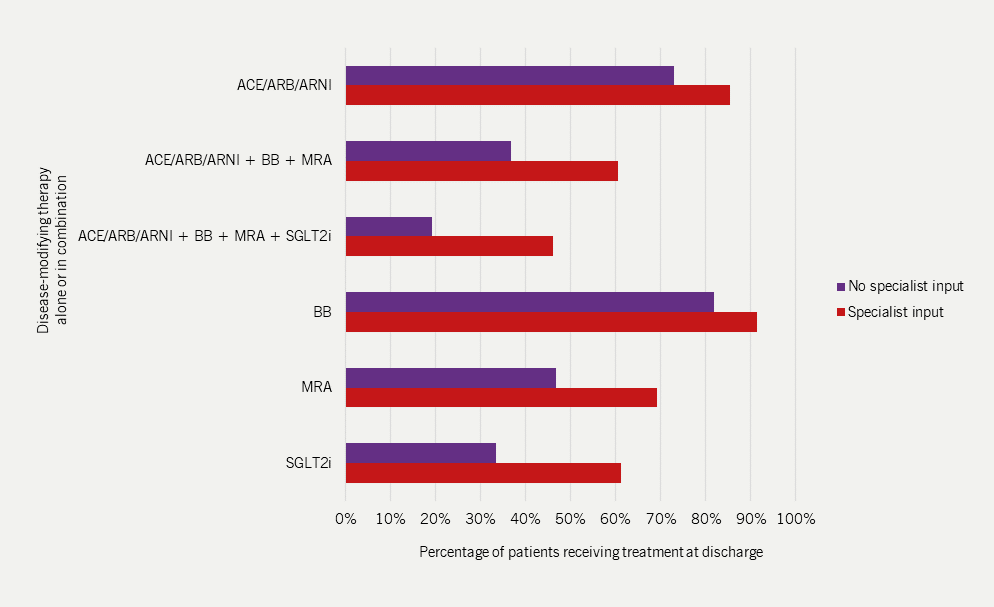
| Adapted with permission from NICOR’s National Heart Failure Audit 2024 Summary Report5 Key: ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; ARNI = angiotensin receptor-neprilysin inhibitor; BB = beta blocker; MRA = mineralocorticoid receptor antagonist; SGLT2i = sodium-glucose co-transporter-2 inhibitor |
The rate of referral to cardiac rehabilitation has fallen
The rate of referral to cardiac rehabilitation after discharge was under 10% in 2022/23; down 3% from the previous year. This may be because the Quality and Outcomes Framework indicators no longer reward practices for referring patients to an exercise-based cardiac rehabilitation programme, despite recommendations made in both European Society of Cardiology and NICE chronic HF guidelines. This is perhaps a reflection of service-underfunding and the so-called ‘postcode lottery’; exercise-based cardiac rehabilitation can improve symptoms and reduce the risk of HF hospitalisation in patients with HFrEF,24,25 but access to such programmes varies greatly nationwide.
Quality standards
NICE has produced ‘quality standards’ for acute and chronic HF that define the standard of care that should be provided.
Acute HF: The NICE acute HF quality standards – updated 201526 – recommend that:
- Adults presenting to hospital with new, suspected acute HF have a single measurement of natriuretic peptide
- Adults admitted to hospital with new, suspected acute HF and raised natriuretic peptide levels have a transthoracic Doppler 2D echocardiogram within 48 hours of admission
- Adults admitted to hospital with acute HF have input within 24 hours of admission from a dedicated specialist HF team
- Adults with acute HF due to left ventricular systolic dysfunction (LVSD) are started on, or continue with, beta-blocker treatment during their hospital admission
- Adults admitted to hospital with acute HF and reduced LVEF are offered an ACE inhibitor and a MRA
- Adults with acute HF have a follow up clinical assessment by a member of the community – or hospital-based specialist HF team within two weeks of hospital discharge.
Chronic HF: The NICE chronic HF quality standard – updated 202327 – recommend that:
- Adults presenting in primary care with suspected HF have their N‑terminal pro‑B‑type natriuretic peptide (NT‑proBNP) measured
- Adults with suspected HF have specialist assessment and transthoracic echocardiography within two weeks of referral if they have a very high NT‑proBNP level, or six weeks if they have a high NT‑proBNP level.
- Adults with chronic HF who have reduced ejection fraction receive all appropriate medication at target or optimal tolerated doses, including:
- ACE inhibitor (or ARB, if ACE inhibitor not tolerated) and/or an ARNI e.g. sacubitril/valsartan
- Beta blocker
- MRA
- SGLT2 inhibitor
- Adults with chronic HF have a review within two weeks of any change in the dose or type of their HF medication
- Adults with stable chronic HF have a review of their condition at least every six months
- Adults with chronic HF receive a personalised programme of cardiac rehabilitation.
The quality standards require significant resources and do not take into account patient variables that may limit their treatment. Many centres struggle to attain these very high standards; quality standards require that all patients should have specialist input from a dedicated specialist heart failure team within the first 24 hours of admission, but only 82% of patients get any input from HF specialists while an inpatient.5 The standards suggest that all patients should get a transthoracic echocardiogram within 48 hours of admission, but only 85% of patients get an echocardiogram at all during admission.
The requirement that all patients have follow-up with either their GP, a cardiologist, or HF specialist nurse within two weeks of discharge must be one of the most unrealistic targets in the modern NHS.
Aetiology, precipitants and comorbidities
Aetiology28
HF is a syndrome and not a diagnosis. Once a patient has been identified as having the HF syndrome, a cause must be sought. For example, in the majority of patients with HFrEF, the underlying cause will be IHD. However, determining the aetiology of HF is often complex, and many causative factors may overlap.
|
National Heart Failure Audit 2024 Report5 The top five causes and comorbidities in both heart failure with reduced and preserved ejection fraction (prevalence, %), respectively, are:
|
Possible aetiologies of HF:
- IHD
- Hypertension
- Valve disease (acquired and congenital)
- Arrhythmias (atrial and ventricular brady- and tachyarrhythmias)
- Dilated cardiomyopathy (DCM)
- Viral
- Idiopathic
- Lyme disease
- Chagas’ disease
- Infective
- Congenital heart disease
- Drug-induced
- Infiltrative
- Storage disorders
- Endomyocardial disease
- Pericardial disease
- Metabolic (autoimmune, nutritional deficiencies, endocrine disease)
- Neuromuscular disease.
Complications and comorbidities
HF is predominantly a disease of the elderly and most patients have multiple comorbidities5; approximately 60% of patients with heart failure have at least one comorbidity.29 Some comorbidities may cause HF in the first place, for example, chemotherapy after treatment for cancer.30 Others are risk factors for developing HF, such as obesity or hypertension (see below). Polypharmacy is common and can be very challenging.
The most common comorbidities include the following:
- Arrhythmias
Arrhythmias are common complications of HF, especially AF (figure 7), which can increase the risk of stroke, thromboembolism and ventricular arrhythmias.31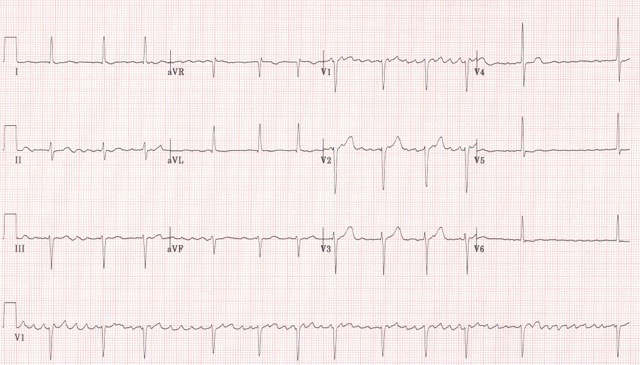
Figure 7. Electrocardiogram showing atrial fibrillation (click to enlarge) - AF
- hyperthyroidism
- electrolyte disorders
- uncontrolled hypertension
- mitral valve disease.
- recent surgery
- chest infection or exacerbation of chronic obstructive pulmonary disease (COPD)/asthma
- acute myocardial ischaemia
- alcohol binge.
Patients with HF who develop AF (figure 7) should be assessed for potentially reversible causes (see below) and stroke risk. Unless there is a very strong contraindication, patients with HF and AF should always be anticoagulated.31
Potential reversible causes of AF in HF
Potential precipitating factors of AF in HF
- Ventricular arrhythmias
Episodes of asymptomatic, non-sustained ventricular tachycardia are common.1 ‘Complex ventricular arrhythmias’, including frequent premature ventricular complexes and non-sustained ventricular tachycardia, are associated with a poor outcome.1
- Anxiety and depression
- Anaemia
- Diabetes
- Chronic kidney disease
- Chronic obstructive pulmonary disease
- Hyperuricaemia and gout
- Hypertension
- Obesity
- Sleep disturbances
Anxiety and depression are common in patients with HF with around one in three patients reporting symptoms of low mood and one in five patients meeting diagnostic criteria for a depressive illness. Depression in patients with HF is associated with a worse clinical status, poor prognosis, poor adherence to treatment and social isolation.32
Chronic HF (CHF) and anaemia frequently co-exist – up to 40% of patients with CHF are anaemic (Hb <13 g/dL in men and <12 g/dL in women).33 The cause of anaemia in patients with CHF is multifactorial34; a combination of disease severity, co-morbidities (such as renal dysfunction), poor diet, haematinic deficiencies and side-effects of treatment. Anaemia in patients with CHF is associated with more severe symptoms and poorer prognoses.35,36
Diabetes affects approximately one third of patients with HF and is associated with a poorer prognosis and reduced functional status.37 Hyperglycaemia is associated with endothelial dysfunction, myocardial fibrosis, and increased activation of the sympathetic nervous system and renin-angiotensin-aldosterone system.38
Chronic kidney disease (CKD) is very common amongst patients with chronic HF – ~50% of patients with CHF have an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2.39 Both CKD and worsening renal function during treatment are associated with a poor prognosis.40 Patients with CHF and CKD have worse symptoms, higher levels of circulating renin-angiotensin-aldosterone system hormones and natriuretic peptides, are more likely to take a diuretic, but are less likely to be treated with an ACE inhibitor/ARB, beta blockers or MRAs.9,41 Despite concerns regarding renal function, treatment with disease-modifying therapies at the expense of renal function is better than the preservation of a ‘normal’ eGFR or creatinine. Additionally, newer drug treatments for HFrEF, such as sacubitril/valsartan and SGLT2 inhibitors, may reduce the rate of decline of renal function.42,43
Chronic obstructive pulmonary disease is another common co-morbidity affecting around 10% of patients but may have negligible impact on prognosis.44 There is much overlap in risk factors and symptoms, prompting concerns about misdiagnosis of a patient with exertional dyspnoea.45 The concern is that a diagnosis of COPD in patients with HFrEF will lead to under-prescription of beta blockers.46
Gout is common in patients with heart failure; diuretics can either cause or aggravate gout.47
Hypertension is more common in patients with HFpEF.31 Among people with hypertension, the incidence of HF is reduced by antihypertensive therapy, with the exception of alpha-adrenoreceptor blockers which are associated with an increased risk and should be discontinued whenever possible.48
Obesity is a risk factor for HF, independent of lifestyle factors that may contribute to, or are associated with being obese, such as physical inactivity, diabetes and hypertension.49 There is potential for misdiagnosis in an obese patient with exertional dyspnoea and ankle swelling. Obesity often makes an echocardiogram challenging and sometimes, uninterpretable.
Disturbed sleep is a common complaint of patients with HF and sleep-disordered breathing affects up to one third.9 Obstructive sleep apnoea is associated with intermittent hypoxaemia, hypercapnia, sympathetic activation, pulmonary and systemic hypertension, and atrial fibrillation.50
Classifications of HF
In classifying HF syndromes, patients were historically grouped into those with acute HF and chronic HF. Various classifications exist, depending on the presentation of the patient, the underlying cause and the pathophysiology particular to that patient. The terminology surrounding HF is sometimes confusing and often unhelpful. Current terms used to classify HF include the following:
- Acute HF versus chronic HF versus acute decompensated/hospitalised HF
- High output versus low output
- Systolic versus diastolic HF
- Right-sided versus left-sided HF
- Phenotype based on LVEF i.e. HFrEF/HRmrEF/HFpEF (See figure 8). This will be discussed in further detail in Module 2.
‘Acute HF’ means different things to different clinicians. To some, it is synonymous with acute pulmonary oedema; to others, it encompasses all patients being admitted to hospital with HF and includes those (the majority with ‘acute’ HF) with fluid retention as their dominant problem.
‘Acute decompensated’ HF is also unsatisfactory – it is not acute (fluid having taken weeks or months to accumulate); nor is it ‘decompensated’ (which implies prior compensation, when it is, in fact, often the presenting feature of HF).
Pulmonary oedema is a dramatic event with an identifiable cause (such as myocardial ischaemia or arrhythmia). It is an uncommon mode of hospitalisation for patients with HF, and requires entirely different treatment from those with severe fluid retention – anasarca. Like patients presenting with pulmonary oedema, those presenting with fluid retention are likely to have an underlying cause.
In this module, we will use the terms ‘acute pulmonary oedema’ and ‘anasarca’ to encompass those patients presenting acutely or subacutely; and ‘chronic HF’ to describe those patients stabilised after an initial presentation and between any exacerbations.
Patients with HF are often sub-divided in relation to their LVEF, which is most commonly measured using transthoracic echocardiography (TTE or ‘echo’).
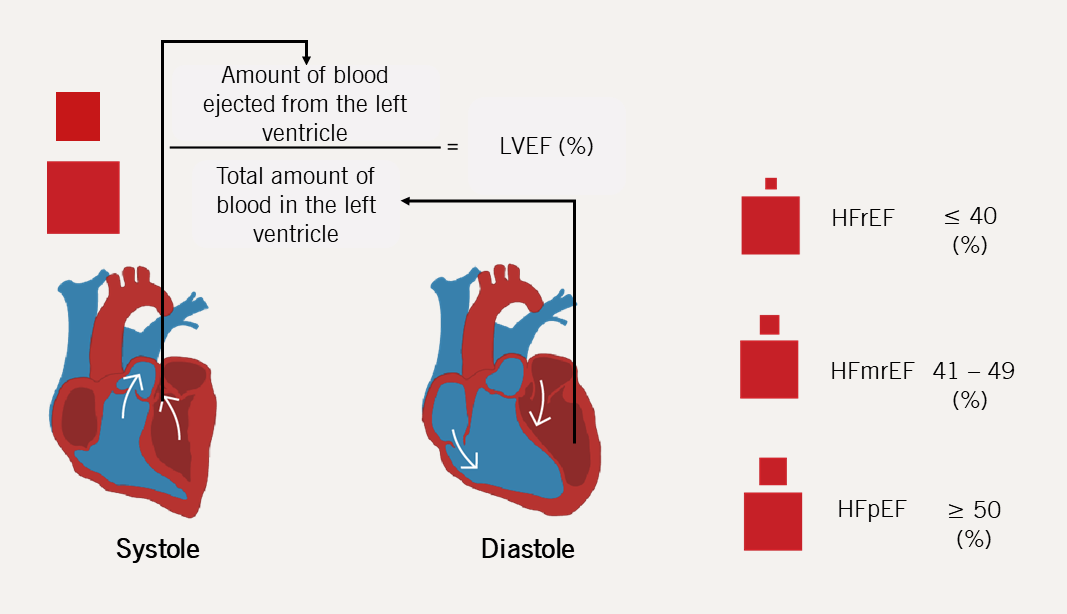
| Key: HFmrEF = heart failure with mildly reduced ejection fraction; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; LVEF = left ventricular ejection fraction |
Despite much debate surrounding the value of LVEF as a measure of cardiac function, there is no doubt that it is helpful in identifying patients who are likely to respond to specific therapies.
Clinical trials have unequivocally demonstrated the benefit of medical and device therapy for patients with HF, particularly amongst those with reduced ejection fraction, whichever way that is defined.
The latest ESC 2023 Focused update to the ESC 2021 Guidelines on the assessment and management of heart failure focuses on the phenotypic classification to determine management.
Syndromes and pathophysiology
As described above, the most useful distinction to make is between decompensated (either with pulmonary oedema or anasarca) and chronic HF. Chronic HF describes the syndrome patients have once decompensated HF has been medically treated.
Pulmonary oedema
Pulmonary oedema is an acute medical emergency, which can be precipitated by the following:
Table 2. Common precipitants of acute pulmonary oedema
| Myocardial ischaemia | Acute infarction Acute coronary syndrome Angina |
| Arrhythmia | Atrial fibrillation Ventricular tachycardia |
| Mechanical disaster | Ventricular septal rupture Papillary muscle rupture |
| Intercurrent illness | Pneumonia Thyrotoxicosis |
| Environmental | Lack of compliance with medication Acute salt loading |
Pathophysiology of pulmonary oedema28
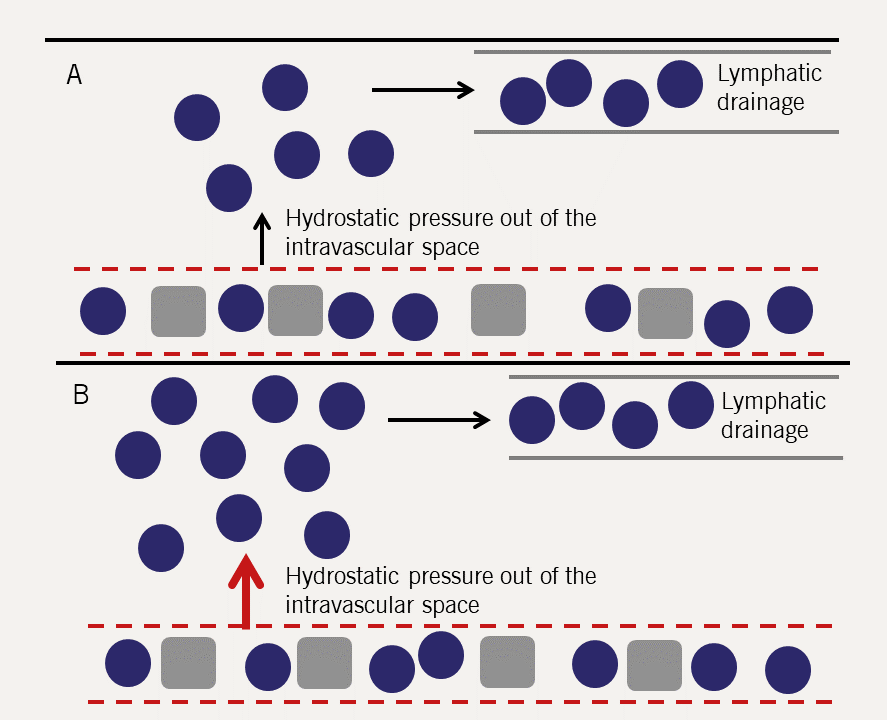
Pulmonary oedema is best understood in haemodynamic terms. Fluid is held in the pulmonary capillaries by the balance between the hydrostatic pressure in the capillary (tends to push fluid out) and the colloid osmotic pressure, which is largely generated by plasma proteins (tending to hold fluid in the vascular space).
There is a net (small) constant transudation of fluid from the pulmonary capillaries which is removed by the lymphatics (figure 9).
Left ventricular work (and cardiac output) is determined by left ventricular filling pressure (end-diastolic pressure). This is the Frank-Starling relationship (figure 10): as filling pressure rises, so does cardiac output. In the acutely failing LV, the relation is shifted downward and to the right so that to maintain any given cardiac output, a higher filling pressure is required.
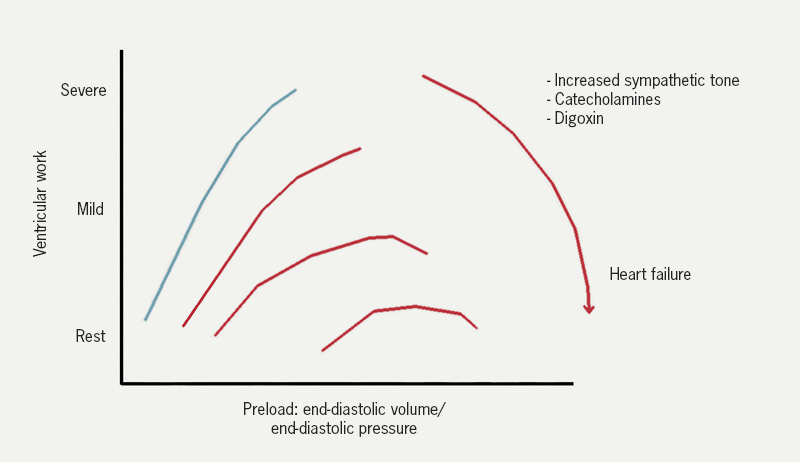
| Adapted McDonagh et al. 2011.9 |
Left ventricular filling pressure is the same as the pulmonary venous pressure (assuming no mitral valve stenosis). As the left ventricle becomes impaired, the end diastolic pressure and pulmonary venous pressure increase. The rise results in an increase in the rate of transudation from the pulmonary capillaries into the lung tissues. The rate of influx eventually exceeds the rate at which fluid can be removed by the lymphatics. Fluid then accumulates in the interstitium of the lung and then the alveoli.
A point often ignored is that the increase in filling pressure requires an input of energy – this can only come from the right ventricle. In acute pulmonary oedema, there must be a temporary imbalance between the output of the two ventricles; the relative excess output from the right results in the increase in left ventricular diastolic pressure and represents the fluid accumulating in the lungs.51
Decompensated HF28
More common than pulmonary oedema as a cause of hospitalisation is fluid retention (table 3). In contrast to patients with pulmonary oedema (who may have a low circulating volume due to accumulation of fluid in the lungs), patients with peripheral oedema have an absolute excess of body fluid and an increased circulating volume. The fluid tends to accumulate gradually over many days or weeks; it takes around 5 L of excess fluid before peripheral oedema appears.
Table 3. Comparison between patients with acute pulmonary oedema and those with peripheral oedema
| Pulmonary oedema | Fluid retention | |
| Onset | Minutes – hours | Days – weeks |
| Precipitant | Always | Rarely |
| Circulating volume | Normal or reduced | Increased |
| Total water volume | Normal | Increased |
| Blood pressure | Increased | Normal or low |
Fluid accumulates with gravity, so for most patients it starts in the ankles and works upward – and may involve the abdominal, and even thoracic, wall. However, patients who are bed bound may have oedema around the sacrum and thighs but little around the feet and ankles. Patients are usually not breathless at rest, although are unable to do much exercise.
On physical examination, the patient may have a tachycardia with a low volume pulse and a low blood pressure. Around 25% will be in AF. The jugular venous pressure is invariably raised, but may be so high that the top of the column of blood cannot be seen, even with the patient sitting upright.
In most patients, the heart is dilated and there is a loud third, and often fourth, heart sound. Some degree of pulmonary venous hypertension is almost invariable and patients may have basal crackles in both lung fields.
Pathophysiology of decompensated HF28
Salt and water are retained by the kidneys in response to decreased renal perfusion, which in turn, leads to renin-angiotensin-aldosterone activation coupled with an increase in antidiuretic hormone production. The increased circulating volume is accommodated largely in the compliant venous side of the circulation; venous hydrostatic pressure thus rises (with gravity ensuring that the hydrostatic pressure is highest in the feet). The capacity of the lymphatics to drain away the transudate is exceeded, and oedema starts to form.
What is less certain is why there is salt and water retention in the first place. It may be related to the primary need for the body to maintain blood pressure. This cannot be the whole explanation, as in some patients, oedema develops despite normal blood pressure and in others, there is fluid retention despite aggressive treatment with neurohormonal antagonists.
Chronic HF28
Although some patients with fluid retention are thought of as having chronic HF – particularly if they do not actually need to be hospitalised – the term is best reserved for patients with treated, decompensated HF.
Successful treatment of acute/decompensated HF leads to chronic HF – patients usually have some degree of exercise limitation – but where HF is well-treated, patients do not have congestion and oedema.
Chronic HF is iatrogenic in the sense that before modern therapies were available, decompensated HF had a very bleak prognosis. Diuretics and treatment with neurohormonal antagonists allow chronic HF to develop.
The cardinal symptom of chronic HF is exercise limitation. Patients complain of breathlessness or fatigue on exertion, with the dominant symptom varying with the kind of exercise performed. The origin of symptoms is not clear and is likely to be a result of abnormal haemodynamics, neurohormonal activation and skeletal muscle changes.
Pathophysiology of chronic HF
- Haemodynamics
In most patients with chronic HF, remodelling causes progressive dilation of the LV. Stroke volume is maintained by an increase in left ventricular wall tension by the law of Laplace. Cardiac output is usually normal at rest and during submaximal exercise, but may not be able to rise normally during more strenuous activity. However, there is little correlation between haemodynamic variables – particularly when measured at rest – and exercise capacity. - Neurohormonal activation
The insight that many neurohormonal systems are activated in chronic HF has been the key to the success of modern medical therapy for HF. - Renin-angiotensin-aldosterone system activation
The renin-angiotensin-aldosterone system (RAAS) (figure 11) is activated by reduced renal perfusion. Plasma concentrations of renin, angiotensin II, and aldosterone are higher in those with worse HF symptoms (higher New York Heart Association [NYHA] class). Diuretics – while essential for controlling symptoms – further increase RAAS activation. The consequences are, inter alia, vasoconstriction, salt and water retention, increased vascular fibrosis and a positive interaction with the sympathetic nervous system (figure 12), which is activated by a fall in cardiac output and RAAS activation. The consequences for the heart include tachycardia, myocardial fibrosis and apoptosis, increased risk of arrhythmia and peripheral vasoconstriction (figure 13). - Natriuretic peptides and arginine vasopressin
Natriuretic peptide concentrations rise as a homeostatic response to dilation of the cardiac chambers. They counteract many of the effects of the RAAS – inducing natriuresis, diuresis and vasodilation. Antidiuretic hormone (arginine vasopressin) is also increased, further enhancing water retention.Other hormone systems are activated, although their clinical relevance is not always clear. The potent vasoconstrictor, endothelin, is raised, and more intriguingly, there is activation of components of the immune system, with increases in tumour necrosis factor and C-reactive protein, for example.
- Skeletal muscle changes
These can be very prominent in some patients resulting in cachexia, but almost all patients have some degree of loss of lean muscle bulk. The skeletal muscle changes correlate closely with impaired exercise capacity, and appear to be implicated, at least in part, as the cause of sympathetic nervous system activation. - Peripheral changes
These include the downregulation of baroreceptors (making them unlikely to be the source of sympathetic nervous system activation) and enhancement of chemoreceptors. The ergoreflex, a neurally mediated reflex arising from exercising muscle in proportion to work done, is increased.
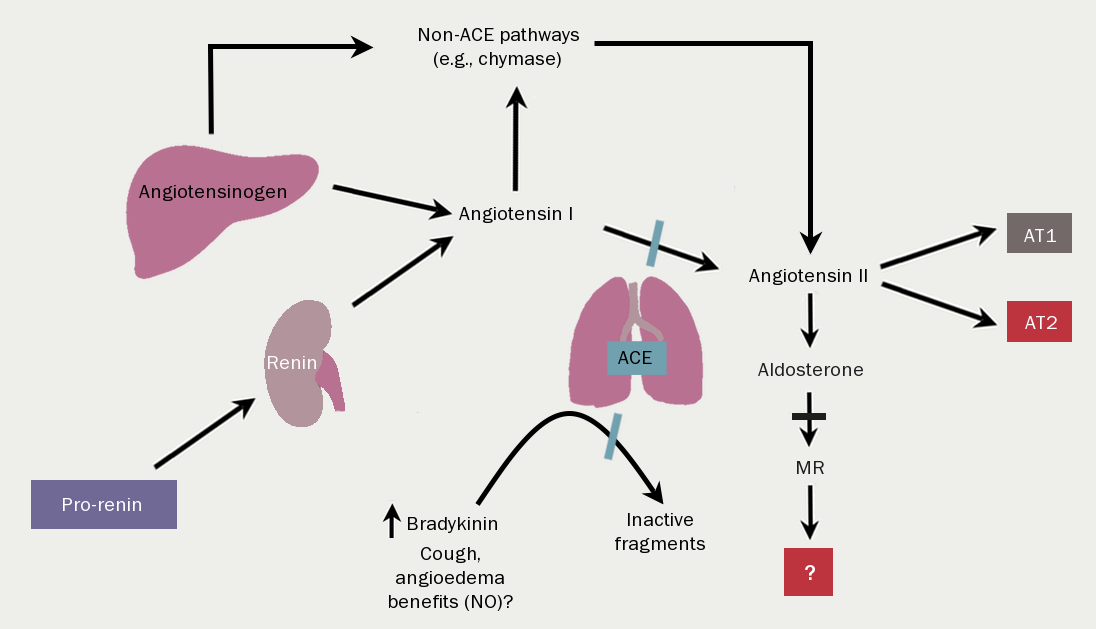
| Key: ACE = angiotensin-converting enzyme; AT1/2 = angiotensin receptor type 1 or 2; MR = mineralocorticoid receptor; NO = nitrous oxide |
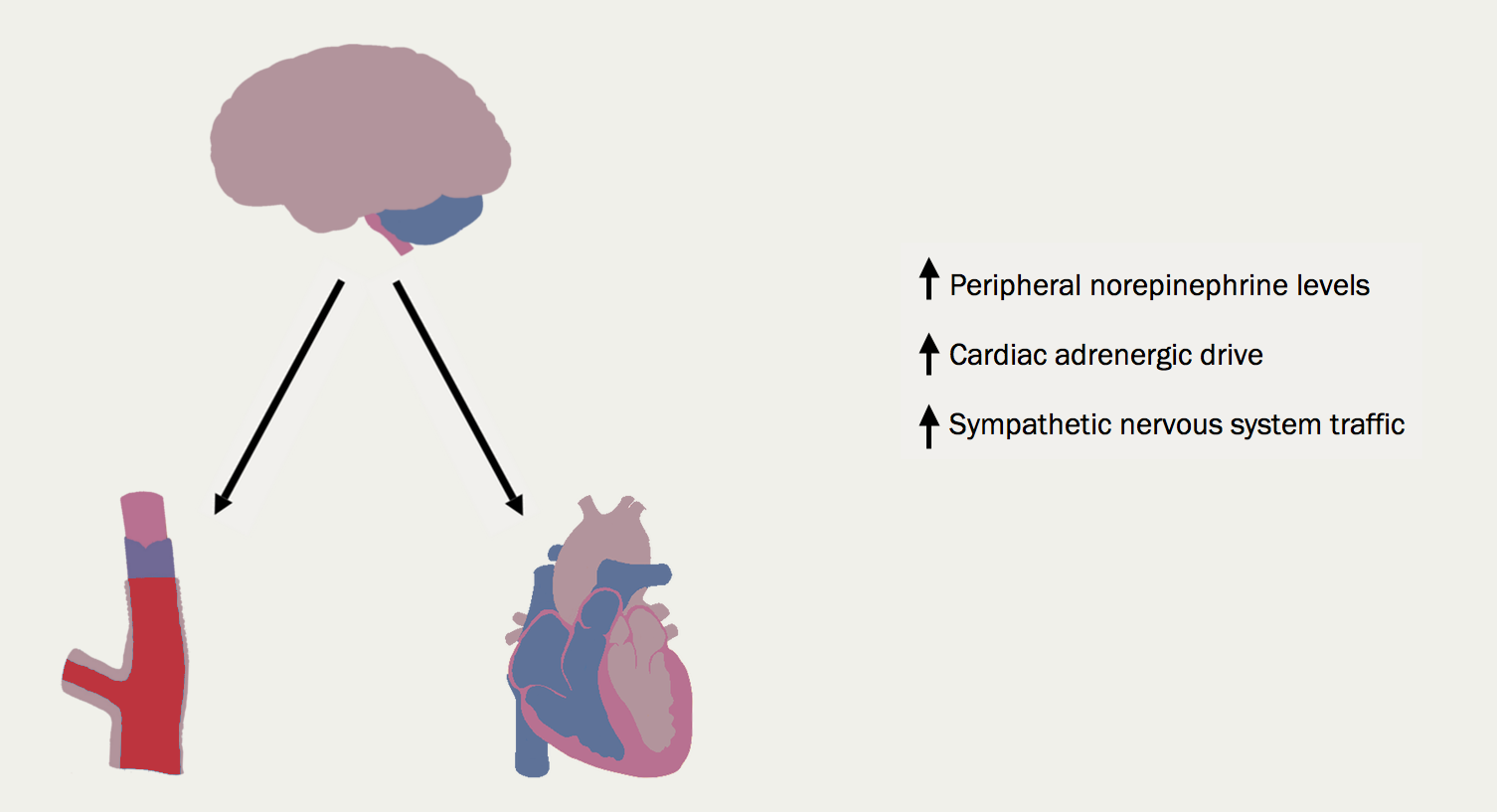
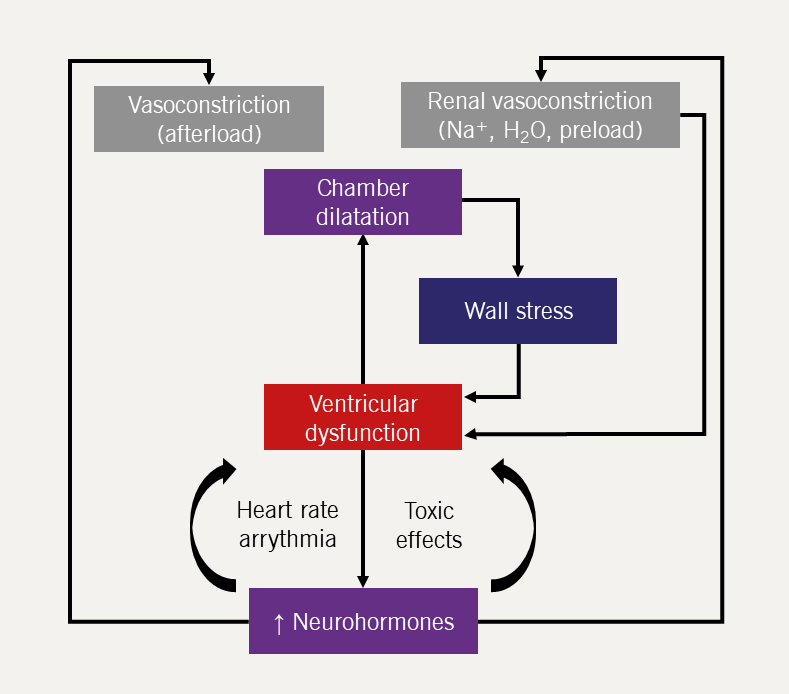
Conclusion
HF has a profound impact on quality of life, prognosis and survival of those affected. Establishing a clear diagnosis can be difficult, especially given the broad and vague diagnostic criteria relating to HFpEF. Its incidence and prevalence rise with age, and both are rising in the population due to better healthcare and therapeutic advances. A large proportion of the healthcare budget is spent on HF hospitalisations.
The pathophysiology of HF remains key to understanding how to diagnose and manage the different types of HF accordingly.
In the following modules in this programme, we will explore investigations, diagnostic algorithms and pharmacological and non-pharmacological options for the management of HF.
close window and return to take test
References
- Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 2016;18:891–975. http://dx.doi.org/10.1002/ejhf.592
- Shahim B, Kapelios CJ, Savarese G, Lund LH. Global public health burden of heart failure: an updated review. Cardiac Failure Review 2023;9:e11. https://doi.org/10.15420/cfr.2023.05
- Conrad N, Judge A, Tran J et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 2018;391:572–80. https://doi.org/10.1016/S0140-6736(17)32520-5 [Epub ahead of print]
- Gerber Y, Weston SA, Berardi C, et al. Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: a community study. Am J Epidemiol 2013;178:1272–80. https://doi.org/10.1093/aje/kwt109 [Epub ahead of print]
- National Institute for Cardiovascular Outcomes Research. National Heart Failure Audit 2024 Summary Report (2022/23 data). Available from: https://www.nicor.org.uk/publications/ncap/heart-failure-1/2024-3/hf-final-report-2022-23?layout=default (accessed 01 May 2024)
- Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJV. More ‘malignant’ than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail 2001;3:315–22.
- Bottle A, Kim D, Aylin P, Cowie MR, Majeed A, Hayhoe B. Routes to diagnosis of heart failure: observational study using linked data in England. Heart 2018;104:600–5. https://doi.org/10.1136/heartjnl-2017-312183 [Epub ahead of print]
- Office for National Statistics. Deaths registered in England and Wales: 2022. London: United Kingdom Government, 2023. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsregistrationsummarytables/2022#leading-causes-of-death [accessed 28 April 2023]
- McDonagh TA, Gardner RS, Clark AL, Dargie H (eds.). Oxford Textbook of Heart Failure. Oxford: Oxford University Press, July 2011. http://dx.doi.org/10.1093/med/9780199577729.001.0001
- Smolina K, Wright FL, Rayner M, Goldacre MJ. Determinants of the decline in mortality from acute myocardial infarction in England between 2002 and 2010: linked national database study [published correction appears in BMJ 2013;347:f7379]. BMJ 2012;344:d8059. https://doi.org/10.1136/bmj.d8059
- Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010;362:2155–65. https://doi.org/10.1056/NEJMoa0908610
- Sutherland K. Bridging the quality gap: heart failure. 2010. The Health Foundation. Available from https://www.health.org.uk/publications/bridging-the-quality-gap-heart-failure/ [accessed 20 October 2023]
- NHS England. 2019/20 General Medical Services (GMS) contract Quality and Outcomes Framework (QOF). Summary of QoF Indicators 2019-20. Available from: https://www.england.nhs.uk/wp-content/uploads/2019/05/gms-contract-qof-guidance-april-2019.pdf (accessed 20 October 2023)
- NHS Digital. Quality and outcomes framework (QOF) – 2019-20. Available at: https://digital.nhs.uk/data-and-information/publications/statistical/quality-and-outcomes-framework-achievement-prevalence-and-exceptions-data/2019-20 [accessed December 2023]
- Cuthbert JJ, Gopal J, Crundall-Goode A, Clark AL. Are there patients missing from community heart failure registers? An audit of clinical practice. Eur J Prev Cardiol 2019;26:291–8. https://doi.org/10.1177/2047487318810839 [Epub ahead of print]
- National Institute for Health and Care Excellence. Acute heart failure: diagnosis and management [CG187]. Last updated 2021. Available at: https://www.nice.org.uk/guidance/cg187 (accessed 20 October 2023)
- British Heart Foundation. Heart Failure hospital admissions rise by a third in five years. 2019. Available from: https://www.bhf.org.uk/what-we-do/news-from-the-bhf/news-archive/2019/november/heart-failure-hospital-admissions-rise-by-a-third-in-five-years [accessed 20 October 2023]
- Martin GP, Kwok CS, Van Spall HGC et al. Readmission and processes of care across weekend and weekday hospitalisation for acute myocardial infarction, heart failure or stroke: an observational study of the National Readmission Database. BMJ Open 2019;9:e029667. https://doi.org/10.1136/bmjopen-2019-029667
- Department of Health. Payment by Results guidance for 2010–11. Leeds: Payment by Results team. 2013. Available from: http://webarchive.nationalarchives.gov.uk/20130105041537/http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/@ps/documents/digitalasset/dh_112970.pdf (accessed 20 October 2023)
- Conrad N, Judge A, Canoy D et al. Temporal trends and patterns in mortality after incident heart failure: a longitudinal analysis of 86,000 individuals. JAMA Cardiol 2019;4:1102–11. https://doi.org/10.1001/jamacardio.2019.3593
- NHS England. 2023/25 NHS Payment Scheme – a consultation notice. Annex DpC: Guidance on best practice tariffs. 2022. Available from: https://www.england.nhs.uk/wp-content/uploads/2022/12/23-25NHSPS_Annex-DpC-Best-practice-tariffs.pdf (accessed 20 October 2023)
- Taylor CJ, Ryan R, Nichols L, Gale N, Hobbs FR, Marshall T. Survival following a diagnosis of heart failure in primary care. Fam Pract 2017;34:161–8. https://doi.org/10.1093/fampra/cmw145
- Sidney S, Go AS, Jaffe MG, Solomon MD, Ambrosy AP, Rana JS. Association between aging of the us population and heart disease mortality from 2011 to 2017. JAMA Cardiol 2019;4:1280–6. https://doi.org/10.1001/jamacardio.2019.4187
- Taylor RS, Sagar VA, Davies EJ et al. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev 2014;2014:CD003331. https://doi.org/10.1002/14651858.CD003331.pub4 [Update in: Cochrane Database Syst Rev 2019;1:CD003331]
- O’Connor CM, Whellan DJ, Lee KL et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1439–50. https://doi.org/10.1001/jama.2009.454
- National Institute for Health and Clinical Excellence. Acute heart failure quality in adults [QS103]. London: NICE, 2015. Available from https://www.nice.org.uk/guidance/QS103 [accessed 20 October 2023]
- National Institute for Health and Clinical Excellence. Chronic heart failure in adults [QS9]. London: NICE, 2023. Available from https://www.nice.org.uk/guidance/qs9 [accessed 20 October 2023]
- Clark AL, Gardner RS, McDonagh TA (eds.). Oxford Textbook of Heart Failure (edn 2) Oxford Textbooks in Cardiology: Oxford Academic, 1 May 2022. https://doi.org/10.1093/med/9780198766223.001.0001
- British Heart Foundation. UK Factsheet. 2023. Available at: https://www.bhf.org.uk/-/media/files/for-professionals/research/heart-statistics/bhf-cvd-statistics-uk-factsheet.pdf [accessed November 2023]
- Loosen SH, Roderburg C, Curth Ole et al. The spectrum of comorbidities at the initial diagnosis of heart failure a case control study. Sci Rep 2022;12:2670. https://doi.org/10.1038/s41598-022-06618-5
- McDonagh TA, Metra M, Adamo M et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure [published correction appears in Eur Heart J 2021 Oct 14]. Eur Heart J 2021;42:3599–726. https://doi.org/10.1093/eurheartj/ehab368
- Celano CM, Villegas AC, Albanese AM, Gaggin HK, Huffman JC. Depression and anxiety in heart failure: a review. Harv Rev Psychiatry 2018;26:175–84. https://doi.org/10.1097/HRP.0000000000000162
- Masini G, Graham FJ, Pellicori P et al. Criteria for iron deficiency in patients with heart failure. J Am Coll Cardiol 2022;79:341–51. https://doi.org/10.1016/j.jacc.2021.11.039
- Witte KK, Clark AL. Micronutrients and their supplementation in chronic cardiac failure. An update beyond theoretical perspectives. Heart Fail Rev 2006;11:65–74. https://doi.org/10.1007/s10741-006-9194-4
- Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur. Heart J 2013;34:816–29. https://doi.org/10.1093/eurheartj/ehs224 [Epub ahead of print]
- Cleland JGF, Zhang J, Pellicori P et al. Prevalence and outcomes of anemia and hematinic deficiencies in patients with chronic heart failure. JAMA Cardiol 2016;1:539–47. https://doi.org/10.1001/jamacardio.2016.1161
- Lehrke M, Marx N. Diabetes mellitus and heart failure. Am J Med 2017;130(Suppl 6):S40–S50. https://doi.org/10.1016/j.amjmed.2017.04.010
- Voors AA, van der Horst ICC. Diabetes: a driver for heart failure. Heart 2011;97:774–80. https://doi.org/10.1136/hrt.2009.183624
- Xiao HB. Echocardiography in primary care. In McIntyre H (ed.). Heart failure in older patients. London: Concise Clinical Consulting, 2007
- Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol 2004;43:317–27. https://doi.org/10.1016/j.jacc.2003.07.046
- Ferrari R, Böhm M, Cleland JGF et al. Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail 2015;17:665–71. https://doi.org/10.1002/ejhf.304
- Bhatia RS, Tu JV, Lee DS et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 2006;355:260–9. https://doi.org/10.1056/NEJMoa051530
- He KL, Burkhoff D, Leng WX et al. Comparison of left ventricular structure and function in Chinese patients with heart failure and ejection fractions >55% versus 40 to 55% versus <40%. Am J Cardiol 2009;103:845–51. https://doi.org/10.1016/j.amjcard.2008.11.050
- Steinberg BA, Zhao X, Heidenreich PA et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction. Prevalence, outcome and therapies. Circulation 2012;126:65–75. https://doi.org/10.1161/CIRCULATIONAHA.111.080770
- Solomon SD, Anavekar N, Skali H et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005;112:3738–44. https://doi.org/10.1161/CIRCULATIONAHA.105.561423
- Fonarow GC, Stough WG, Abraham WT et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure. J Am Coll Cardiol 2007;50:768–77. https://doi.org/10.1016/j.jacc.2007.04.064
- Kapoor JR, Kapoor R, Ju C et al. Precipitating clinical factors, heart failure characterization, and outcomes in patients hospitalized with heart failure with reduced, borderline, and preserved ejection fraction. J Am Coll Cardiol 2016;4:464–72. https://doi.org/10.1016/j.jchf.2016.02.017
- Gottdiener JS, McClelland RL, Marshall R et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med 2002;137:631–9. https://doi.org/10.7326/0003-4819-137-8-200210150-00006
- Pascual-Figal DA, Ferrero-Gregori A, Gomez-Otero I et al. Mid-range left ventricular ejection fraction: Clinical profile and cause of death in ambulatory patients with chronic heart failure. Int J Cardiol 2017;240:265–70. https://doi.org/10.1016/j.ijcard.2017.03.032 [Epub ahead of print]
- Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017;69:841–58. https://doi.org/10.1016/j.jacc.2016.11.069
- McIver DH and Clark AL. The vital role of the right ventricle in the pathogenesis of acute pulmonary edema. Am J Cardiol 2015;115(7):992–1000. https://doi.org/10.1016/j.amjcard.2015.01.026

All rights reserved. No part of this programme may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publishers, Medinews (Cardiology) Limited.
It shall not, by way of trade or otherwise, be lent, re-sold, hired or otherwise circulated without the publisher’s prior consent.
Medical knowledge is constantly changing. As new information becomes available, changes in treatment, procedures, equipment and the use of drugs becomes necessary. The editors/authors/contributors and the publishers have taken care to ensure that the information given in this text is accurate and up to date. Readers are strongly advised to confirm that the information, especially with regard to drug usage, complies with the latest legislation and standards of practice.
Healthcare professionals should consult up-to-date Prescribing Information and the full Summary of Product Characteristics available from the manufacturers before prescribing any product. Medinews (Cardiology) Limited cannot accept responsibility for any errors in prescribing which may occur.