Introduction
Although left ventricular (LV) systolic function recovers in around 15% of patients with heart failure due to a reduced ejection fraction (HFrEF) within three years with optimal medical treatment (OMT),1 the same proportion will progress to end-stage disease and surgical intervention is sometimes necessary.2
Surgical treatment for heart failure (HF) includes:
- mechanical circulatory support
- LV assist devices (LVAD)
- cardiac transplantation
- coronary revascularisation
- surgical ventricular restoration
- valve surgery.
|
As many as one-quarter of patients with end-stage HF may have an unrecognised need for advanced therapies.3 |
While identifying the need for mechanical circulatory support in a patient with cardiogenic shock may be easy enough, identifying ambulatory patients with chronic HF who require advanced therapy (such as LVAD or transplantation) is more difficult.
Donor organ shortage is a frequently cited reason for the low rate of heart transplantation in the UK – according to Eurotransplant (a non-profit organisation that co-ordinates organ transplantation across continental Europe) there are approximately twice as many patients awaiting heart transplant per year than those who receive one.4
Mechanical circulatory support
LVADs
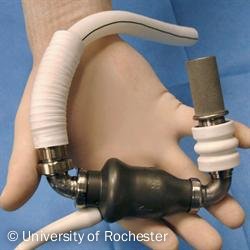
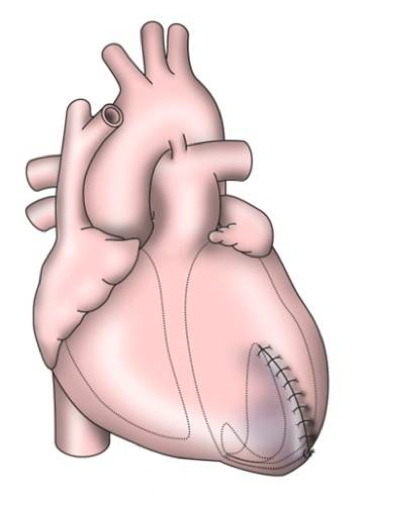
Mechanical circulatory support (MCS) was developed as a rescue therapy for patients in intractable cardiogenic shock. The first devices were conceived as a continuation for patients who were unable to come off cardiopulmonary bypass (CPB) and have progressed to separate, implantable LVADs (figure 1).
Historically, LVADs were only indicated as a holding measure until an organ became available or until a decision was made regarding cardiac transplantation; so-called ‘bridge-to-transplantation’ (BTT) or ‘bridge-to-decision’ (BTD).
However, in 2015, the National Institute for Health and Clinical Excellence (NICE) published guidelines also recommending LVADs as long-term therapy for patients ineligible for transplantation, so-called ‘destination therapy’ (DT).5
In America, LVADs are licenced for a third indication known as ‘bridge-to-candidacy’, whereby the LVAD is implanted in a patient ineligible for heart transplantation with the hope that the improvement in haemodynamics may enable the patient to become eligible for transplant at a later date.
LVADs are used as DT more commonly than other indications worldwide (figure 2).6 In a minority of patients, the haemodynamic relief provided by the LVAD can allow recovery of myocardial function and explant of the device, though the majority will require continued support.
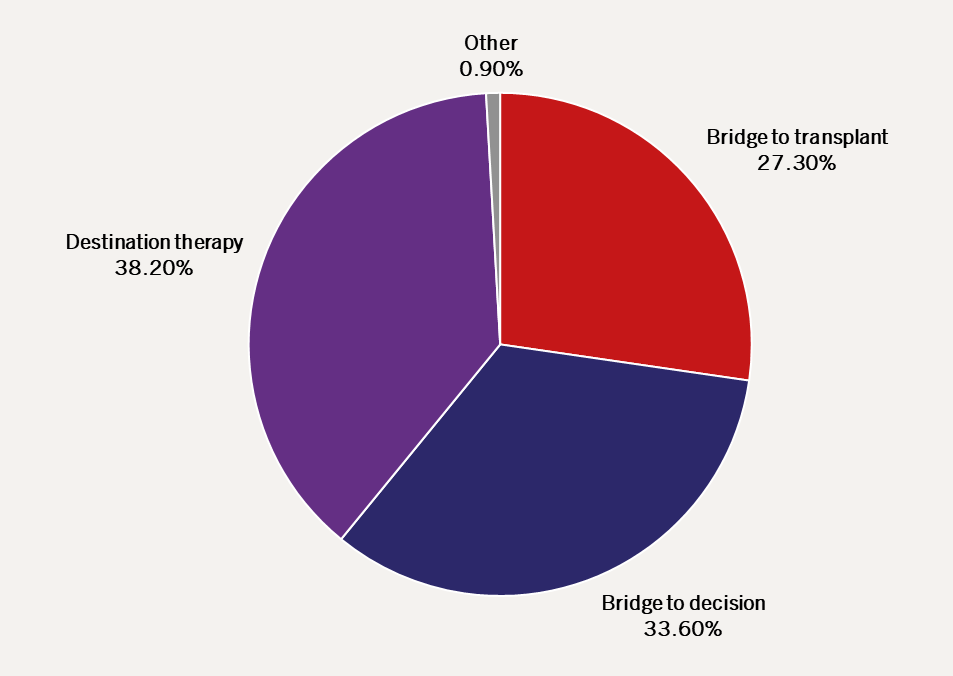
| Adapted from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS)6 |
The standard LVAD circuit pumps blood from the LV cavity through the device into the ascending aorta, creating a mechanical bypass of the LV outflow. The left ventricle is thus off-loaded (and the aortic valve may remain closed). Cardiac output is thus increased.7
Beneficial effects of LVAD include:
- increased systemic blood pressure
- improved organ perfusion and subsequent beneficial neuro-hormonal changes
- reduced cardiac chamber size
- reduced left atrial and pulmonary pressures.
Development of LVADs8
The first generation of LVADs (e.g. HeartMate® I) were large, pulsatile displacement pumps. The haemodynamic benefits were offset by significant morbidity from extensive surgical dissection required for a large device, high rates of infection due to the high calibre driveline, and limited durability necessitating reoperation.
The second-generation pumps (e.g. HeartMate® II, Jarvik 2000®) are smaller devices with impeller pumps that provide continuous flow compared to biphasic flow of first-generation devices. In head-to-head trials with first-generation technology, the HeartMate® II device was associated with higher survival rates at two years free of disabling stroke or reoperation to replace the device (46% vs. 11%; p<0.001; HR, 0.38; 95% CI, 0.27–0.54) possibly due to the smaller size.9
Various third-generation devices are now in use or undergoing further evaluation. An example is the HeartMate® III, which has a miniaturised centrifugal pump that uses magnetic and hydrodynamic forces to levitate and rotate the impeller, theoretically avoiding wear on bearings. The smaller device size allows for quick and less-invasive implantation, though the adverse event rate remains stubbornly high – see ‘Evidence‘ below.
Complications of LVAD therapy10
|
Despite developments in design, the morbidity associated with LVAD therapy continues to impede progress in the field; 70% of patients with either continuous or pulsatile LVAD suffer a first episode of infection, bleeding, device malfunction, stroke or death within one year.10 |
The most frequent problems encountered during LVAD therapy are:
- Bleeding and thrombosis
- All patients require anticoagulation, with the specific regime varying between devices
- All patients also develop acquired von Willebrand factor deficiency
- The tiny pulse pressure with continuous flow pumps is associated with gastrointestinal bleeding due to the development of angiodysplasia (much like in severe aortic stenosis)
- Device thrombosis is a difficult problem manifested by increased power consumption, clinical or laboratory signs of haemolysis (e.g. elevated bilirubin, plasma haemoglobin and lactate dehydrogenase levels) or device stoppage. It can be treated with intravenous heparin, fibrinolytics or surgery (pump exchange or urgent transplant).
- Infection
- The risk of infection is high; the artificial surfaces of the pump itself create sites for colonisation and the percutaneous driveline that delivers power is an extremely common site of infection
- Severity of infection can vary from asymptomatic driveline site colonisation to deep abscesses or device-related endocarditis. Wound care requires specialist nursing oversight.
- Right ventricular failure
- This is precipitated by failure of the right ventricle (RV) to handle the volume returned to it by the assisted LV (particularly because the right ventricle can be involved in the underlying disease process)
- This can be manifested by distended neck veins, liver congestion and increasing oedema, accompanied by a deterioration in exercise capacity and increasing natriuretic peptides
- Treatment is with diuretics, adjustment of the LVAD speed, or insertion of a right-sided ventricular assist device (VAD) in extreme cases.
- Stroke
- Both types of stroke – ischaemic due to embolism or haemorrhagic due to bleeding – are often catastrophic, if they occur
- Haemorrhagic stroke is especially feared; anticoagulation cannot be stopped due to the high likelihood of device thrombosis and failure.
- Acquired aortic regurgitation
- LVAD therapy creates a large pressure gradient from the aorta to the LV, transmitted across the aortic valve; this can lead to aortic regurgitation, which decreases the efficacy of support and longevity of the device
- Up to 50% will have moderate to severe aortic regurgitation at 18 months, which is difficult to treat. Options include open or transcatheter valve replacement or cardiac transplantation.
- Arrhythmia
- Both atrial and ventricular arrhythmia are common in patients with LVAD. Patients with LVADs are unique in regard to ventricular arrhythmia in that an episode of VF does not lead to death as peripheral blood flow is maintained by the pump. Cardioversion is still required!
Evidence
High adverse event rates are inevitable in severely unwell and unstable patients, such as those suitable for LVAD therapy. Trials of LVADs in patients with less severe disease have had mixed results.
REVIVE-IT (Randomised Evaluation of VAD Intervention Before Inotropic Therapy), a randomised controlled trial of LVADs in patients who were less ill than patients currently eligible for destination therapy (New York Heart Association [NYHA] class II–IV with ‘advanced HF’ defined by markers such as hyponatraemia or high natriuretic peptide), was discontinued because of the high adverse event rate.11
In the ROADMAP (Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients) study, investigators enrolled 200 patients (average age, 64 years; median B-type natriuretic peptide [BNP], 547 pg/ml in the LVAD arm) who were eligible for LVAD therapy with a second-generation device (HeartMate® II).12 Treatment was assigned per patient wishes to either LVAD implantation or OMT. The primary end point was survival on original therapy with >75 metre improvement in six-minute walk distance at one year. More patients in the LVAD group met the primary end point, with the difference increasing at two-years’ follow up (30% vs. 12%; p=0.012).12,13 In an as-treated analysis, LVAD implantation was associated with a better one-year survival (80% vs. 63%; p=0.02), but because 30% of patients in the OMT group had LVAD implantation over two years, there was no significant difference in intention-to-treat analysis.12,13 However, while the results are promising, it is essential to bear in mind treatment with an LVAD in the ROADMAP study was not randomised; almost a third of patients in the ‘control’ arm changed their mind and opted for an LVAD during the two-year study period. As a result, the data are compromised.
In the ENDURANCE trial (Intrapericardial Left Ventricular Assist Device for Advanced Heart Failure), investigators enrolled 446 patients (average age, 64 years; NYHA III–IV; average left ventricular ejection fraction (LVEF), 17%), all of whom were eligible for LVAD therapy but ineligible for heart transplantation (i.e. the LVAD was a ‘destination therapy’ or ‘bridge to candidacy’ – common indications for implantation in America but not in the UK).14 Patients were randomised to either the commonly used HeartMate® II device with an axial rotating pump implanted in the abdominal cavity, or the HeartWare™ HVAD™ with a smaller, magnetically levitated pump implanted in the pericardium.14 The absence of bearings in the magnetically levitated pump was hypothesised to reduce the risk of device failure, thrombus formation and stroke.
The primary end point was a composite of survival free from disabling stroke, urgent transplantation or device explant or replacement due to complications two years after implantation.14 The primary end point was similar between the two devices, suggesting non-inferiority of the HeartWare™ HVAD™ compared to the HeartMate® II device.14 However, adverse event rates were high in both arms. While patients with the HeartMate® II device were more likely to require device replacement, explantation or urgent transplantation (16% vs. 9%; p=0.03), they were less likely to have a stroke (12% vs. 30%; p<0.001), right HF (27% vs. 39%; p=0.02) or sepsis (15% vs. 24%; p=0.05) compared to the HeartWare™ HVAD™.15
In the very similar MOMENTUM 3 trial (Multicenter Study of MagLev™ Technology in Patients Undergoing Left Ventricular Assist Device Therapy with HeartMate 3™), investigators enrolled 294 patients with end-stage HF who were randomised to either the HeartMate® II device or the HeartMate® III device (similar to the HeartWare™ HVAD™ device with a magnetically levitated centrifugal pump that is implanted in the pericardial space).15 More patients in the HeartMate® III arm met the primary end point of survival free of disabling stroke or device explant or replacement due to complications six months after implantation (86% vs. 77%; p<0.001), suggesting non-inferiority (p<0.001).15 Although the study was not powered to detect superiority, the HeartMate® III did appear to be the better device (p=0.04).15 Adverse event rates were again high but similar in the two groups.15
A retrospective cohort analysis comparing outcomes amongst patients in the UK implanted with the HeartMate® II device or the HeartWare™ HVAD™ device found no significant difference in outcome or adverse event rates between the devices.16
In the UK, data on LVAD implantation is collected by the NHS Organ Donation and Transplant Service which releases annual activity reports. In 2021/22, there were 334 LVAD implantations for long-term bridging.17 The median time on an LVAD was 1,101 days.17 The majority of patients were listed for a heart transplant within a year of LVAD implantation but only 5% of patients underwent transplantation within that time frame. While outcome has improved, it remains poor – more patients die on support (31%) than are transplanted (13%) or have the device explanted due to recovery (5%).17
The future of LVADs lies not only in developing technology to reduce the morbidity and cost of long-term therapy, but also in their appropriate deployment. Many patients are referred too late in the natural history of HF (or when there is serious co-morbidity) and thus outcomes are poor and the only viable long-term treatment – transplantation – is often contraindicated. The timing of LVAD insertion remains critical. The economic argument will only carry weight when the up-front cost is a great deal lower. No publicly funded health care system can afford the present costs of a device in even larger numbers of patients than at the moment.
Cardiac transplantation
Cardiac transplantation has evolved significantly since its inception in the 1960s and remains the best long-term therapy for advanced HF, despite its risks and complications (figure 3).18
| Data obtained from NHS BTCAG-H, 202218 |
Clinically, the key challenges are management of patient selection, donor organ selection, the peri-operative period and long-term follow up, in addition to availability of donor organs. Practically, the key challenge is shortage of available donor organs, which limits the expansion of the transplant population.19
By the end of March 2023 in the UK, there were 254 adult patients on the heart transplant waiting list.17 Between 2018 and 2020 amongst 302 patients on the non-urgent transplant list, 20% had been transplanted within three years of listing, whilst 27% were still waiting, 29% had progressed to the urgent list and 10% had died while waiting.17
Patient selection19
|
The aim is to identify those patients with a sufficiently poor prognosis to justify the increased early mortality risk with better long-term survival being the reward. |
Good cardiac transplant candidates have advanced and severely symptomatic HF, but do not have other end-organ complications or co-morbidities associated with high peri-operative risk or which adversely affect long-term outcome. In-patient referral may be considered for patients admitted with acute HF with intractable inotrope dependence or cardiogenic shock.19,20
Box 1. Clinical features that should prompt consideration for transplant
| >2 admissions with acute HF in the last 12 months |
| Persistent signs and symptoms of HF despite maximal therapy |
| Evidence of right ventricular dysfunction or increasing pulmonary arterial pressure (aim to refer before mean pulmonary artery pressure is >50 mmHg) |
End-organ damage that is attributable to HF and may be correctable with transplant:
(Aim to refer before estimated glomerular filtration rate <40 ml/min/1.73 m2) |
| Key: HF = heart failure |
Box 2. Criteria for heart transplantation
| Left ventricular systolic dysfunction not due to a correctable cause, such as valve disease |
| NYHA class III or IV |
| Optimal medical and device therapy (if indicated) |
Evidence of a poor prognosis:
|
| Key: HF = heart failure; NYHA = New York Heart Association |
Box 3. Criteria for urgent in-patient referral
| Persistent cardiogenic shock due to primary cardiovascular cause |
| Continuous intravenous inotropic support |
| Mechanical circulatory support |
| Ongoing coronary ischaemia with no revascularisation treatment options |
| No contraindication to transplantation. |
Box 4. Absolute contraindications to heart transplantation
Primary end-organ damage not secondary to advanced HF:
|
Pulmonary hypertension:
|
| Active infection or sepsis (other than in the case of an infected LVAD21) |
| Recent pulmonary embolism |
| Microvascular complications of diabetes other than non-proliferative retinopathy |
| Primary end-organ damage not secondary to advanced HF |
| Key: HF = heart failure; LVAD = left ventricular assist device |
Box 5. Relative contra-indications to heart transplantation
Obesity:
|
| Active malignancy other than localised non-melanoma skin cancer |
| Symptomatic peripheral or cerebrovascular disease |
| Auto-immune disease |
Infiltrative cardiac disease:
|
| Severe skeletal myopathy with associated cardiomyopathy |
| Smoking |
| Excessive alcohol use |
| History of non-adherence to treatments |
Transplant assessment is summarised in table 1.20,22
|
Patients who undergo transplantation require life-long follow up, monitoring and treatment – a stable home life with adequate social support and psychological evaluation are also important aspects of patient selection.11 |
Table 1. Summary of transplant assessment
| Assessment | Key findings | |
| Heart failure aetiology and current therapy | ● Standard heart failure assessment with history, clinical examination and targeted investigations | ● Establish aetiology of heart failure and correct precipitants where possible ● Ensure medical and device therapy are optimised |
| Heart function and prognosis | ● NYHA functional class ● Serum levels of natriuretic peptides ● Echocardiogram +/- cardiac MRI ● 6 minute walk test ● Cardiopulmonary exercise test ● Cardiac catheterisation including coronary angiogram and right heart study |
● Meeting criteria for transplantation – Severe symptoms despite maximal medical/device – Indicators of poor prognonsis, for example low maximal oxygen uptake on exercise testing, or significantly elevated natriuretic peptides |
| Complications of heart failure, particularly renal impairment, liver congestion, anaemia, and pulmonary hypertension | ● Serum electrolytes and renal function ● 24 hour urine for protein and creatinine clearance ● Liver enzymes ● Full blood count and coagulation screen |
● Potential contraindications to transplantation – Irreversible pulmonary hypertension – Irreversible renal failure – Liver dysfunction |
| Other co-morbidities or risk factors with implications for long-term outcome | ● Smoking status ● Chest radiograph and lung function tests ● Autoimmune screen ● Targeted screens for malignancy based on symptoms ● Psychological assessment |
● Potential contraindications to transplantation – Active malignancy – Substance use e.g. smoking or excess alcohol – Respiratory failure – Autoimmune disorders – Inadequate social support |
| Consideration of allograph immunology | ● Blood group ● HLA screen ● Serology screen (includes hepatitis B and C viruses, human immunodeficiency disease, varicella zoster virus, cytomegalovirus, toxoplasma and syphilis ● Screen for other infection (e.g. blood cultures, urine cultures, skin swabs, dental assessment, MRSA screen) |
● Planning organ selection to minimise risk of immune incompatibility ● Potential contraindications to transplantation – Ongoing acute or chronic infection – High levels of HLA sensitisation |
| Key: HLA = human leukocyte antigen; MRI = magnetic resonance imaging; MRSA = methicillin-resistant Staphylococcus aureus; NYHA = New York Heart Association | ||
|
The Organ Donation (Deemed Consent) Act 2019 became law from 15th March 2020 in England, Wales and Northern Ireland, meaning that all adults are presumed to have given consent for organ donation, unless there is a documented decision otherwise. The act may increase the availability of organs for heart transplantation. |
Donor organ selection and peri-operative management19
Specialised teams based at cardiac transplant centres carry out organ retrieval. Techniques for selecting suitable organs are complex and usually involve clinical examination, electrocardiography (ECG), haemodynamic studies using a pulmonary artery catheter, and direct visual inspection (figure 4). This is complemented by data from echocardiography or cardiac catheterisation, if available. The organ is transferred to the centre in cold storage or in an organ care system that maintains a warm, perfused state, where available.
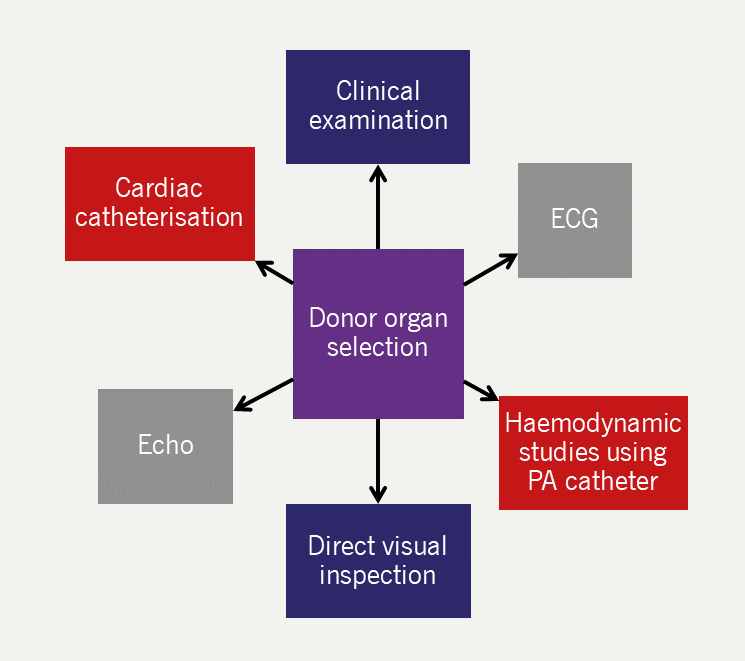
| Key: ECG = electrocardiography; Echo = echocardiography; PA = pulmonary artery |
Performing a heart transplant
The surgical transplant operation has evolved over time.22 The bi-atrial technique left some recipient right atrium in situ and required long, sometimes fallible atrial anastomoses (figure 5a). The total orthotopic technique involved separate anastomoses for each pulmonary vein and the great vessels (not pictured). The bicaval technique involved the insertion of the recipient’s pulmonary veins into the left atrium remains in situ, with the donor left atrium anastamosed to the remnant and the donor right atrium is anastamosed to the great veins (figure 5b).
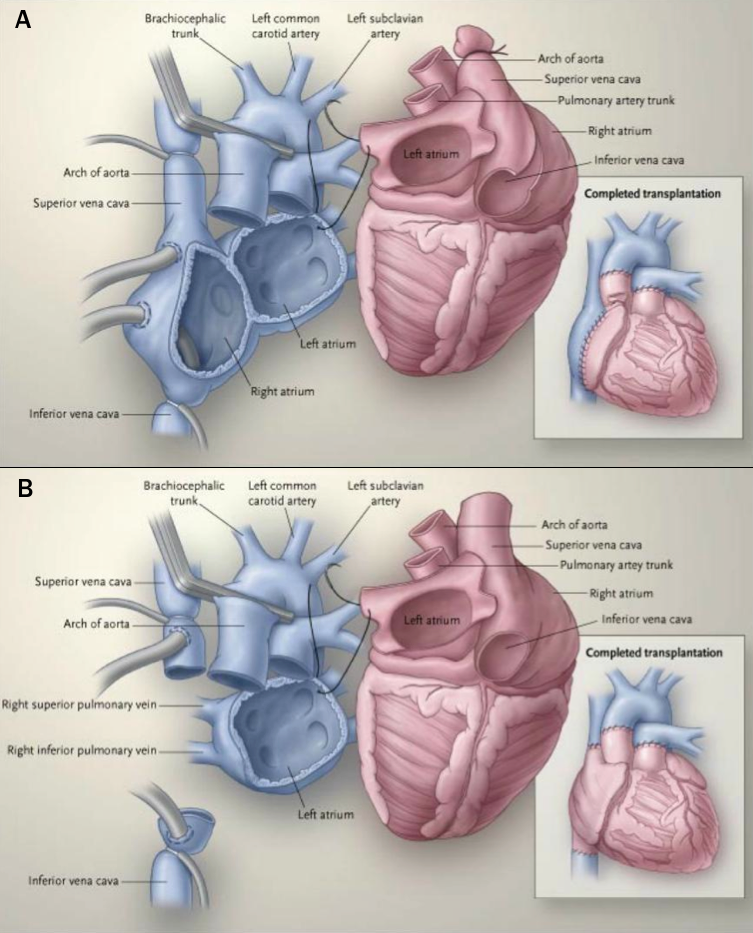
| Reproduced with kind permission from Hunt22 |
The operation and post-operative recovery can vary from the routine (such as in a stable patient with a first sternotomy) to extremely high-risk (such as in patients with several previous sternotomies, a VAD in situ, or in patients who are critically ill). Specific early problems that should be anticipated are summarised in table 2.
Table 2. Problems to anticipate following cardiac transplantation19
| Right ventricular failure |
|---|
| Right ventricular function is particularly susceptible to ischaemia and this becomes aparent after ceasing CPB. This can be compounded by high recipient pulmonary vascular resistance and multiple blood transfusion |
| Bradyarrhythmia |
| Usually sinus bradycardia or sinus arrest. Most centres use expectant temporary atrial pacing or isoprenaline to maintain an early relative tachycardia. This overcomes the initially low stroke volume that normalises with improving ventricular compliance |
| Systemic inflammatory response |
| This particularly occurs with prolonged CPB or pre-existing infection (e.g. chronic LVAD infection), often requiring high-dose vasopressors |
| Haemorrhage |
| Frequently this is due to coagulopathy rather than a surgical cause. Pericardial bleeding can be exacerbated by the presence of potential space after removal of the enlarged heart, or by size mismatch of the donor organ |
| Biventricular failure |
| In the early phase, this raises concern for primary graft failure and hyperacute rejection |
| Key: CPB = cardiopulmonary bypass; LVAD = left ventricular assist device |
Long-term management
Cardiac transplant dramatically improves survival and quality of life (QoL) in the right patient. Based on the NHS’ annual report on heart transplantation in the UK published in 2023, the national rate of patient survival following adult heart transplant at one and five years is 85.9% and 71.4% respectively.17
However, morbidity rates are high.23 The crux of follow-up lies in careful management of immunosuppression, finding the balance between avoidance of rejection and avoidance of side effects of therapy.
The immunosuppressant therapies all target T-cells, which drive cellular rejection. Induction immunosuppression given pre-operatively is sometimes included. The calcineurin inhibitors, tacrolimus and ciclosporin, are the mainstay of therapy and are often combined with corticosteroids and a cell-cycle agent, such as mycophenolate, for long-term maintenance. The drugs have many side effects, many of which are dose-dependent making monitoring of drug levels mandatory.24
Cardiac allograft vasculopathy (CAV) is the most common late complication (weeks to months from transplantation). It is an accelerated form of coronary artery disease in a transplanted heart. As only around 10–30% of patients regain any innervation to the heart post-transplant, angina symptoms are uncommon and patients often present with breathlessness following silent myocardial infarction, or with HF, or as sudden death.25 CAV is often diffuse and therefore not amenable to revascularisation. Careful symptomatic (anti-anginals) and preventative (statins) medical therapy is a priority; most patients are started on low-dose atorvastatin (10 mg once daily) due to the lower incidence of myositis compared with other statins.26
Box 6. Key factors to consider in long-term follow up
Anticipation and early treatment of allograft rejection:
|
| Monitoring for evidence of chronic allograft dysfunction with surveillance echocardiography |
| Stringent monitoring of drug levels and renal function is essential to prevent nephropathy. Kidney disease can be exacerbated by other factors such as pre- or peri- transplant renal injury, coexistent diabetes and hypertension |
| Careful management of hypertension and hyperlipidaemia; both common pre-morbid diagnoses that can be caused or exacerbated by immunosuppressive drugs. Cardiac denervation may also contribute to hypertension. Together, these can increase the risk of CAV |
| Prompt treatment of infection, due to ongoing susceptibility with immunosuppression, and the possibility of superadded bone marrow suppression due to the drugs |
| Monitoring for immunosuppression-associated malignancy, particularly non-melanoma skin tumours and B-cell lymphomas |
| Key: CAV = cardiac allograft vasculopathy; HLA = human leukocyte antigen; LV = left ventricular |
Revascularisation, ventricular restoration and valve surgery
Patients with HF are commonly considered for coronary revascularisation and valve surgery. Surgical ventricular restoration (SVR) involves exclusion of scar on the ventricular wall from previous myocardial infarction and is not commonly performed. Such decisions are difficult, not least because patients with HF often have multiple co-morbidities, including frailty, which makes the long-term benefit of invasive treatment less certain. However, there may be a subset of patients who benefit from certain treatments. As ever, patient selection and shared decision-making is key.27
Revascularisation
Inadequate myocardial perfusion can cause impaired contractility; this can be either fixed (infarction leading to scar) or reversible (hibernation). Differentiating hibernating myocardium from infarcted tissue can be done, for example with dobutamine stress echocardiography or thallium scintigraphy. Coronary revascularisation in HF aims to improve myocardial function by restoring perfusion to hibernating myocardium, so reducing LV volumes and improving LV function (figures 6a and 6b).


Evidence
Until publication of the STICH (Surgical Treatment for Ischaemic Heart Failure) trial results in 2011, the practice of revascularising hibernating myocardium was common but based on anecdotal evidence.
The STICH trial (n=1,212; median age, 59 years; NYHA I–IV; median LVEF, 27% in the treatment arm) was designed to test whether revascularisation improved outcomes in patients with HF and underlying coronary disease without angina severe enough to warrant revascularisation on its own merits.28 There was no significant difference in all-cause mortality between patients treated by revascularisation with coronary artery bypass grafting (CABG) plus OMT and those treated with OMT alone.28 There was no requirement for patients to undergo functional testing before entering the trial and thus the investigators were unable to differentiate between hibernating or infarcted myocardium.28
The STICH extension (STICHES) was published in 2016.29 The original patient cohort from STICH was followed for almost 10 years and at that point, there was a small, but definite, survival advantage for the surgically treated group.29 It is important to note that the patients in STICH had a mean age just short of 60 years and that it took a very long time for the survival advantage to appear.29
The HEART trial (Heart Failure Revascularisation Trial) (n=138; average age, 65 years, two-thirds of whom were NYHA I–II; median wall motion index 0.8 [equivalent to LVEF of 24%]) was a randomised trial of OMT versus OMT plus angiography with the intent to revascularise either via percutaneous coronary intervention (PCI) or CABG.30 Unlike STICH, all eligible patients underwent perfusion testing and the presence of myocardial viability was an inclusion criterion.30 However, like STICH, there was no difference in mortality between the OMT group and the revascularisation group.30 The study was closed early due to slow recruitment, sponsor withdrawal and the start of the STICH study – the results are underpowered as a result.30
The REVIVED-BCIS-2 trial (Revascularization for Ischemic Ventricular Dysfunction- British Cardiovascular Intervention Society-2) (n=700; average age, 70 years; 77% NYHA class I/II, 66% of whom had no angina; mean LVEF, 27%; median NT-proBNP, 1,376 ng/L) tested the hypothesis that PCI in patients with HFrEF due to ischaemic cardiomyopathy may improve outcomes.31 Although superficially similar to the STICH study, patients had to have extensive coronary artery disease and demonstrable myocardial viability in segments supplied by vessels that were amenable to PCI.28,31 The primary end point was hospitalisation for HF or all-cause mortality.31 After a median follow up of 41 months, PCI had no effect on the primary end point (37% vs. 38%) or any of its component parts, regardless of the extent of viability, nor did PCI have any effect on LVEF measured in a sub-set of participants at six and 12 months.31
There is a reasonable argument that selected younger patients with HF (in whom angina is not a prominent symptom) should be offered coronary angiography with a view to surgical revascularisation. There is no role for PCI for the treatment of LV systolic dysfunction, even in the presence of so-called viable myocardium.
Surgical ventricular restoration27
The aims of surgical ventricular restoration (SVR) are to:
- normalise ventricular geometry
- improve myocardial perfusion
- reduce LV work
- improve LV contraction.
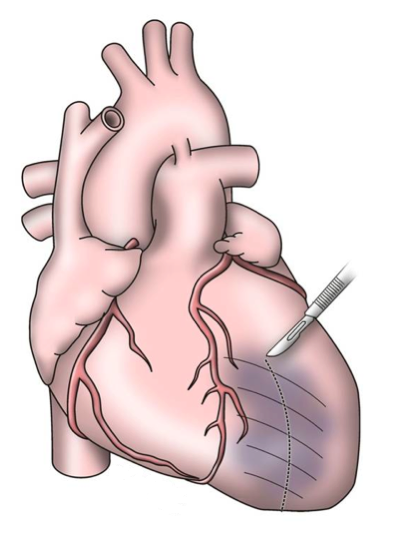
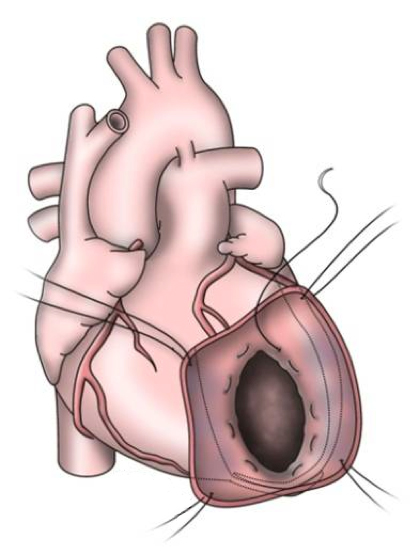
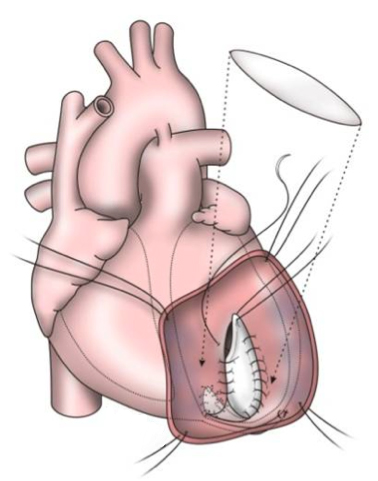
The early procedures were similar to partial left ventriculectomy for aneurysm, while the contemporary SVR involves resection of the non-functional myocardium before placement of an endoventricular Dacron patch to exclude the scar (figures 7a–d). It is usually done during CABG for patients with ischaemic HF and anterior wall akinesia or severe dyskinesia. It adds about 20 minutes to the procedure time without increasing operative risk.
Evidence
In non-randomised studies, SVR improves LVEF and other haemodynamic variables.27 However, hypothesis 2 of the STICH trial compared outcomes of patients randomised to CABG plus SVR to CABG alone and found no significant difference in the composite end point of all-cause mortality or cardiovascular hospitalisation.
Percutaneous ventricular restoration has also been investigated: the Parachute device, a percutaneously delivered device that partitions the LV as a means of ventricular restoration, appears safe and feasible in patients with HF and LV dilatation.33 However, a phase III trial was terminated in June 2017.34
There may yet be sub-groups who do benefit from surgical or percutaneous ventricular restoration and, similar to revascularisation, this book is not yet closed.
Valve surgery
An important cause of the HF syndrome is valvular heart disease. Every patient presenting with HF should be assessed for valvular disease; aortic stenosis is a particularly common cause. Appropriate patients should be offered aortic valve replacement as a potentially curative procedure. Many patients, however, may be too frail for surgical valve replacement by the time they present with HF.
In these cases, transcatheter aortic valve implantation (TAVI) should be considered as being a lower-risk alternative.35 TAVI is certainly superior to medical therapy in inoperable patients and is increasingly being shown in trials to be as effective as surgical valve replacement in lower-risk patients.35
A more difficult problem is that of mitral valve surgery. Functional mitral regurgitation (MR) is very common in HF as a consequence of valve ring dilation, together with tethering of the posterior leaflet of the valve.36
Evidence
There are two randomised controlled trials of the MitraClip® device in patients with HFrEF and MR with conflicting results.
In the COAPT study (Cardiovascular Outcomes Assessment of the MitraClip® Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation), investigators enrolled 614 patients with LVEF <50% and moderate–severe or severe functional MR (average age, 72 years; ~90% NYHA II–III; average NT-proBNP, 5,174 ng/L; average LVEF, 31%; 51% severe MR) who were randomised to either transcatheter mitral valve repair with the MitraClip® device or OMT.37 The primary end point was HF hospitalisations with 24 months follow up. Of the 302 patients in the treatment group, 287 successfully underwent device implantation.37 Treatment with the device was associated with a lower risk of hospitalisation with HF (36% per patient-year vs. 68%; p<0.001) and lower mortality (a non-powered secondary end point; 18% vs. 22%, p<0.001) compared to medical treatment alone.37
In the MITRA-FR study (Percutaneous Repair with the MitraClip® Device for Severe Functional/Secondary Mitral Regurgitation), investigators enrolled 304 patients with LVEF <40% and severe functional MR (average age, 70 years; 91% NYHA II–III, median NT-proBNP, 3,407 ng/L; average LVEF, 33%) who were randomised to mitral valve repair with the MitraClip® device or OMT.38 The primary outcome was all-cause mortality or hospitalisation with HF. Of the 152 patients in the treatment group, 138 had the device successfully implanted.38 There was no difference in the primary end point between the groups after 12 months (55% vs. 51%; p=0.51).38
It is not clear why two very similar trials have yielded two very different results. One explanation may be that medical supervision of the control group was far more strict and the number and changes in medication were greater in the MITRA-FR trial than the COAPT study.39 For example, despite the high number of hospitalisations with HF in the COAPT study, there were no significant changes in HF medications (including diuretics) during the study period; it may be that mitral valve repair in patients with HFrEF is beneficial only when compared to inadequate medical management.39 Another favoured explanation is that the patients in COAPT had smaller LV volumes for a given severity of MR, implying that they had MR out of proportion to their LV impairment. They were, therefore, more likely to respond to mitral intervention.39
The benefit of the MitraClip® in COAPT was maintained at five-year follow up,40 but perhaps the most important finding of the COAPT trial is just how unwell patients were. More than 90% of patients in the control arm had been hospitalised with HF or died after five-year follow up, and despite the benefit of the MitraClip®, 73% of patients in the treatment arm met the primary end point. Mortality rates were 67% and 57% in the control and treatment arms respectively after five years.40 The presence of severe MR in patients with HF should prompt discussion about prognosis, and the goals of treatment, including MitraClip®.
How transcatheter mitral valve repair will fit into future guidelines is not clear, but it is certain that, as with all surgical treatment options for HF, appropriate and timely patient selection will be key to effective use.
Summary
Revascularisation, either by CABG or PCI, is commonly performed with patients with LV systolic dysfunction. However, there is little evidence that it influences prognosis outside of an acute coronary syndrome. If HF is thought to be due primarily to valve disease, then treatment of the valve is likely to benefit the patient only if they are fit for the intervention; patient selection for surgical or transcatheter valve interventions is difficult, but key to success.
Palliative care in HF
|
Palliative care is an approach that improves the QoL of patients and their families |

|
At any stage of the disease, patients with HF have poorer QoL than the general population,41,42 largely due to the significant symptom burden which worsens as the disease progresses.43 In those patients where surgical intervention is not appropriate, or in end-stage disease, end-of-life and palliative care should be implemented.
There is very little data on the benefits of palliative care in patients with HF. A recent meta-analysis of all randomised controlled trial data involving 921 patients with HF found that palliative care interventions were associated with an improvement in QoL and symptom burden, and reduced hospitalisations compared to usual care. The European Society of Cardiology (ESC) and NICE HF guidelines recognise the need for supportive and palliative care services for patients with end-stage HF.44–46
Integrated working between primary care, HF services and specialist palliative care is essential but a palliative approach to care and access to palliative care is patchy, both nationally and internationally.47
Barriers to effective palliative care in patients with HF include:
- unpredictable disease trajectory
- poor communication between members of the multidisciplinary team (MDT) – for example, a lack of clarity about the appropriate ceiling of medical therapy
- reluctance of clinical staff to have the difficult conversations around the end of life due to concerns about destroying hope
- healthcare services are poorly set up for timely and effective palliative interventions.
What is palliative care?
|
Table 3. World Health Organisation definition of palliative care47
|
||||||||||
|
Palliative care (table 3) aims to improve the QoL of patients with chronic or terminal illness and help their families face the problems associated with life-threatening illness. The primary goal is the prevention and relief of suffering by early identification, assessment and treatment of pain and/or psychological distress, as well as addressing spiritual needs.
Palliative care for patients with HF is provided by an MDT led by a palliative care specialist, in conjunction with a HF specialist, with access to other relevant services for persistent or complex needs. It involves a problem-based integrated approach to advanced care planning, communication and symptom control. In order to explore the palliative care of patients with HF, each aspect will be addressed in turn.
There are several common misunderstandings about palliative care. These include:
- a belief that palliative care is only applicable to patients for whom there is clearly apparent irreversible deterioration with death expected within days to months
- requires a handover of the patient to the palliative care team
- only relates to physical symptom control in the dying patient and can only be provided once all other therapeutic options are exhausted.
Problem-based integrated approach
Educating the patient, carers and families about the condition allows for informed joint decision-making. This should be an ongoing process across multiple clinical encounters; the unpredictable trajectory of HF means constant reassessment of a patients’ risk of adverse outcomes and, therefore, potential need for palliative care services is essential.
The level of palliative care intervention at different stages in a patient’s disease process will vary from patient-to-patient, dependent on their needs. Most patients can be cared for in primary care and by their usual secondary care teams, but some require access to specialist palliative care services. This integrated approach allows patients to receive palliative care depending upon need rather than prognosis.48,49
|
Discussions about the appropriate ‘ceiling of care’ are essential to avoid unnecessary hospital admissions or futile treatment – both of which ultimately may cause harm to the patient. |
Important considerations in patients with HF receiving palliative care include:
- Frailty and malnutrition
- Advance care planning
- Communication
- Symptom control
Regardless of how they are defined, frailty and malnutrition are common in HF, affecting over 50% of patients.50,51 Patients with frailty and/or malnutrition have more severe disease; are less likely to be treated with disease-modifying drugs; and are at greater risk of hospitalisation or death.51–54 The majority of hospitalisations or deaths occurring in frail patients are due to non-cardiovascular causes.52 Indeed, in all patients with chronic HF – whether frail or not – non-cardiovascular causes account for the majority of morbidity and mortality in the last year of life.55
All disease-modifying therapies for HFrEF are given on the basis that they reduce HF hospitalisation and cardiovascular mortality.46 Thus, the presence of frailty or malnutrition (or the suspicion that the patient may be in or near the last year of life – see below) should prompt discussions about discontinuing HF therapies, especially if the patient is experiencing side effects.
Advance care planning is defined by the General Medical Council as “the process of discussing the type of treatment and care that a patient would or would not wish to receive in the event that they lose capacity to decide or are unable to express a preference.”50
Advance care planning is important to many patients; it increases the likelihood of end-of-life wishes being carried out and improves the quality of care for both patients and their carers.51–54
One aspect of advance care planning is decision-making about preferred place of care. In the UK, there is a gross disparity between preferred place of care for cancer patients and HF patients.56 Palliative care services tend to be structured to meet the needs of patients with cancer, many of whom have a predictable disease trajectory; as a consequence, most patients will die at home or in a hospice. In contrast, patients dying from cardiovascular diseases, including HF, are much more likely to die in hospital (59%) with fewer than 1% dying in a hospice.55,57
For example, decisions regarding deactivation of an implantable cardioverter-defibrillator (ICD) in a patient predicted to be in the last few weeks of life can be particularly difficult but are an essential aspect of care; if an ICD is not re-programmed to pacemaker mode only, dying may be complicated by repeated shocks.58
Guidance and training have been developed to help physicians and the many patients with HF who want to discuss end-of-life issues.59,60 Patients are involved in decisions about their future care, despite uncertain disease trajectories by “hoping for the best, and preparing for the worst.”61–66 Such discussions should be ongoing through the natural history of disease and not reserved for when a patient displays clear signs of end-stage disease. Regarding ICDs, it is good practice to inform the patient that the time may come at which treatment with an ICD is no longer appropriate as part of counselling before implantation.
Breathlessness (figure 8) is the predominant symptom of end-stage HF. Decisions on investigations and treatment must be taken in the context of the patient’s wishes and stage of the disease.67
For example, intravenous (IV) diuretics, vasodilators and inotropes are not appropriate for someone in the last few days of life whose primary wish is to be kept comfortable in whom less aggressive treatments are essential.
|
Box 7. Palliative management of breathlessness68
|

|
|
Despite its widespread use, there is no evidence to support the use of home-based oxygen as a treatment for breathlessness.75 Home oxygen therapy for patients with NYHA III or IV class symptoms has no impact on QoL measures or survival.76 |
Pragmatic approach to HF treatment
Many medications used to treat HF lower blood pressure or heart rate, yet confer prognostic benefit. Postural hypotension is common among elderly patients77 and is associated with an increased risk of falls and injury.78 In patients with HF who are approaching the end of their lives who often require high-dose oral diuretics, continuing high-dose medications such as beta blockers, ACE inhibitors and mineralocorticoid receptor antagonists may cause more harm than long-term benefit.
How do we know a patient with HF is nearing the end of their life?
The natural history of HF is defined by periods of relative stability punctuated by periods of unpredictable and often sudden decline from which the patient may, or may not, recover. Thus, distinguishing patients whose conditions are salvageable from those who are entering the last few weeks of life is difficult. The ESC list five criteria, the presence of one or more of which ought to prompt consideration of end-of-life care for patients in whom advanced treatment, such as with heart transplantation or LVADs, has been deemed futile44:
- Progressive functional decline and increasing dependence with activities of daily living
- Severe symptoms and poor QoL, despite optimal HF management
- Frequent hospital admissions or other severe decompensation episodes, despite OMT
- Cardiac cachexia
- Clinically judged to be end-of-life for another reason, such as severe infection or advanced cancer.
Summary
Incorporation of a palliative care approach in response to patient need rather than estimated prognosis will result in better care for patients with advanced HF. Interventions to support the patient and family with symptom control with the aim of improving QoL should be used alongside optimal HF management. Palliative care should be provided by the usual clinical teams in primary and secondary care, with access to specialist palliative care services when needed for persistent or complex problems.
close window and return to take test
References
- Kalogeropoulos AP, Fonarow GC, Georgiopoulou V et al. Characteristics and outcomes of adult outpatients with heart failure and improved or recovered ejection fraction. JAMA Cardiol 2016;1:510–8. https://doi.org/10.1001/jamacardio.2016.1325
- Kalogeropoulos AP, Samman-Tahhan A, Hedley JS et al. Progression to stage d heart failure among outpatients with stage C heart failure and reduced ejection fraction. JACC Heart Fail 2017;5:528–37. https://doi.org/10.1016/j.jchf.2017.02.020 [Epub ahead of print]
- Lund LH, Trochu JN, Meyns B et al. Screening for heart transplantation and left ventricular assist system: results from the ScrEEning for advanced Heart Failure treatment (SEE-HF) study. Eur J Heart Fail 2018;20:152–60. https://doi.org/10.1002/ejhf.975 [Epub ahead of print]
- Eurotransplant. Statistics Report Library. Yearly Statistics Overview 2022. Available from http://statistics.eurotransplant.org/index.php?search_type=overview&search_text=9023 (accessed 11 January 2024)
- National Institute for Health and Care Excellence. Implantation of a left ventricular assist device for destination therapy in people ineligible for heart transplantation [IPG516]. London: NICE, 2015. Available from: https://www.nice.org.uk/guidance/ipg516 (accessed 31 October 2023)
- Kirklin JK, Naftel DC, Pagani FD et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant 2015;34:1495–504. https://doi.org/10.1016/j.healun.2015.10.003 [Epub ahead of print]
- Tedford RJ, Leacche M, Lorts A, Drakos SG, Pagani FD, Cowger J. Durable Mechanical Circulatory Support: JACC Scientific Statement. J Am Coll Cardiol 2023;82:1464–81. https://doi.org/10.1016/j.jacc.2023.07.019
- Berardi C, Bravo CA, Li S et al. The history of durable left ventricular assist devices and comparison of outcomes: HeartWare, HeartMate II, HeartMate 3, and the future of mechanical circulatory support. J Clin Med 2022;11:2022. https://doi.org/10.3390/jcm11072022
- Slaughter MS, Rogers JG, Milano CA et al. HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009;361:2241–51. https://doi.org/10.1056/NEJMoa0909938 [Epub ahead of print]
- Kirklin JK, Naftel DC, Kormos RL et al. Quantifying the effect of cardiorenal syndrome on mortality after left ventricular assist device implant. J Heart Lung Transplant 2013;32:1205–13. https://doi.org/10.1016/j.healun.2013.09.001 [Epub ahead of print]
- Pagani FD, Aaronson KD, Kormos R et al. The NHLBI REVIVE-IT study: Understanding its discontinuation in the context of current left ventricular assist device therapy. J Heart Lung Transplant 2016;35:1277–83. https://doi.org/10.1016/j.healun.2016.09.002 [Epub ahead of print]
- Estep JD, Starling RC, Horstmanshof DA et al. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: results from the ROADMAP study. J Am Coll Cardiol 2015;66:1747–61. https://doi.org/10.1016/j.jacc.2015.07.075
- Starling RC, Estep JD, Horstmanshof DA et al; ROADMAP Study Investigators. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: the ROADMAP study 2-year results. JACC Heart Fail 2017;5:518–27. https://doi.org/10.1016/j.jchf.2017.02.016 [Epub ahead of print]
- Rogers JG, Pagani FD, Tatooles AJ et al; ENDURANCE Investigators. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med 2017;376:451–60. https://doi.org/10.1056/NEJMoa1602954
- Mehra MR, Naka Y, Uriel N et al; MOMENTUM 3 Investigators. A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med 2017;376:440–50. https://doi.org/10.1056/NEJMoa1610426 [Epub ahead of print]
- Parameshwar J, Hogg R, Rushton S et al. Patient survival and therapeutic outcome in the UK bridge to transplant left ventricular assist device population. Heart 2019;105:291–6. https://doi.org/10.1136/heartjnl-2018-313355 [Epub ahead of print]
- National Health Service. Blood and transplant. Annual report on heart transplantation. Report for 2022/2023. Available from: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/30881/nhsbt-heart-transplantation-report-2223.pdf (accessed 10 January 2024)
- National Health Service. Blood and Transplant Cardiothoracic Advisory Group – Heart. Long-term Conditional Survival Post-Heart Transplant. 2022. Available at: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/30608/item-123-ctagh-22-52-conditional-survival-final.pdf (accessed 10 January 2024)
- Banner NR, Bonser RS, Clark AL et al. UK guidelines for referral and assessment of adults for heart transplantation. Heart 2011;97:1520e1527. https://doi.org/10.1136/heartjnl-2011-300048
- Dar O, Banner NR. Cardiac transplantation: who to refer and when. Br J Hosp Med (Lond) 2013;74:258–63. https://doi.org/10.12968/hmed.2013.74.5.258
- McDonagh TA, Metra M, Adamo M et al; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022;24:4–131. https://doi.org/10.1002/ejhf.2333
- Hunt SA. Taking heart – cardiac transplantation past, present, and future. N Engl J Med 2006;355:231–5. https://doi.org/10.1056/NEJMp068048
- Dew MA, Kormos RL, Roth LH, Murali S, DiMartini A, Griffith BP. Early post-transplant medical compliance and mental health predict physical morbidity and mortality one to three years after heart transplantation. J Heart Lung Transplant 1999;18:549–62. https://doi.org/10.1016/s1053-2498(98)00044-8
- Crespo-Leiro MG, Costanzo MR, Gustafsson F et al. Heart transplantation: focus on donor recovery strategies, left ventricular assist devices, and novel therapies. Eur Heart J 2022;43:2237–46. https://doi.org/10.1093/eurheartj/ehac204
- Aranda JM Jr, Hill J. Cardiac transplant vasculopathy. Chest 2000;118:1792–800. https://doi.org/10.1378/chest.118.6.1792
- Chih S, Chong AY, Mielniczuk LM, Bhatt DL, Beanlands RS. Allograft vasculopathy: the Achilles’ heel of heart transplantation. J Am Coll Cardiol 2016;68:80–91. https://doi.org/10.1016/j.jacc.2016.04.033
- Hegeman RRMJJ, Swaans MJ, van Kuijk JP, Klein P. State-of-the-art review: Technical and imaging considerations in hybrid transcatheter and minimally invasive left ventricular reconstruction for ischemic heart failure. J Clin Med 2022;11:4831. https://doi.org/10.3390/jcm11164831
- Velazquez EJ, Lee KL, Deja MA et al; STICH Investigators. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med 2011;364:1607–16. https://doi.org/10.1056/NEJMoa1100356 [Epub ahead of print]
- Velazquez EJ, Lee KL, Jones RH et al; STICHES Investigators. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med 2016;374:1511–20. https://doi.org/10.1056/NEJMoa1602001 [Epub ahead of print]
- Cleland JG, Calvert M, Freemantle N et al. The heart failure revascularisation trial (HEART). Eur J Heart Fail 2011;13:227–33. https://doi.org/10.1093/eurjhf/hfq230 [Epub ahead of print]
- Perera D, Clayton T, O’Kane PD et al; REVIVED-BCIS2 Investigators. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med 2022;387:1351–60. https://doi.org/10.1056/NEJMoa2206606 [Epub ahead of print]
- Brener MI, Uriel N, Burkhoff D. Left ventricular volume reduction and reshaping as a treatment option for heart failure. Structural Heart 2020;4:264–83. https://doi.org/10.1080/24748706.2020.1777359
- Costa MA, Mazzaferri EL Jr, Sievert H, Abraham WT. Percutaneous ventricular restoration using the parachute device in patients with ischemic heart failure: three-year outcomes of the PARACHUTE first-in-human study. Circ Heart Fail 2014;7:752–8. https://doi.org/10.1161/CIRCHEARTFAILURE.114.001127 [Epub ahead of print]
- US National Institutes of Health. ClinicalTrials.gov. A multinational trial to evaluate the parachute implant system (PARACHUTE). Available from: https://clinicaltrials.gov/show/NCT01286116 (accessed 01 November 2023)
- Prendergast BD, Baumgartner H, Delgado V et al. Transcatheter heart valve interventions: where are we? Where are we going?. Eur Heart J 2019;40:422–40. https://doi.org/10.1093/eurheartj/ehy668
- Clark AL, Alamgir MF, Nair RK, Thackray ST. Percutaneous surgery for mitral valve disease. Heart 2009;95:1850. https://doi.org/10.1136/hrt.2009.167536
- Stone GW, Lindenfeld J, Abraham WT et al; COAPT Investigators. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med 2018;379:2307–18. https://doi.org/10.1056/NEJMoa1806640 [Epub ahead of print]
- Obadia JF, Messika-Zeitoun D, Leurent G et al; MITRA-FR Investigators. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018;379:2297–306. https://doi.org/10.1056/NEJMoa1805374 [Epub ahead of print]
- Grayburn PA, Sannino A, Packer M. Proportionate and disproportionate functional mitral regurgitation: a new conceptual framework that reconciles the results of the MITRA-FR and COAPT Trials. JACC Cardiovasc Imaging 2019;12:353–62. https://doi.org/10.1016/j.jcmg.2018.11.006 [Epub ahead of print]
- Stone GW, Abraham WT, Lindenfeld J et al; COAPT Investigators. Five-year follow-up after transcatheter repair of secondary mitral regurgitation. N Engl J Med 2023;388:2037–48. https://doi.org/10.1056/NEJMoa2300213 [Epub ahead of print]
- Teno JM, Weitzen S, Fennell ML, Mor V. Dying trajectory in the last year of life: does cancer trajectory fit other diseases? J Palliat Med 2001;4:457–64. https://doi.org/10.1089/109662101753381593
- Hobbs FDR, Kenkre JE, Roalfe AK, Davis RC, Hare R, Davies MK. Impact of heart failure and left ventricular systolic dysfunction on quality of life. A cross-sectional study comparing common chronic cardiac and medical disorders and a representative adult population. Euro Heart J 2002;23:1867–76. http://dx.doi.org/10.1053/euhj.2002.3255
- Barclay S, Momen N, Case-Upton S, Kuhn I, Smith E. End-of-life care conversations with heart failure patients: a systematic literature review and narrative synthesis. Br J Gen Pract 2011;61:e49–62. http://dx.doi.org/10.3399/bjgp11X549018
- Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 2016;18:891–975. https://doi.org/10.1002/ejhf.592
- Rogers JG, Patel CB, Mentz RJ et al. Palliative care in heart failure: the PAL-HF randomized, controlled clinical trial. J Am Coll Cardiol 2017;70:331–41. https://doi.org/10.1016/j.jacc.2017.05.030
- McDonagh TA, Metra M, Adamo M et al; ESC Scientific Document Group. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2023;44:3627–39. https://doi.org/10.1093/eurheartj/ehad195 [Erratum in: Eur Heart J 2023 Nov 23]
- Harrison N, Cavers D, Campbell C, Murray SA. Are UK primary care teams formally identifying patients for palliative care before they die? Br J Gen Pract 2012;62:e344–e52. http://dx.doi.org/10.3399/bjgp12X641465
- Sepúlveda C, Marlin A, Yoshida T, Ullrich A. Palliative care: the World Health Organization’s global perspective. J Pain Symptom Manage 2002;24:91–6. http://dx.doi.org/10.1016/S0885-3924(02)00440-2
- Hogg KJ, Jenkins SMM. Prognostication or identification of palliative needs in advanced heart failure: where should the focus lie? Heart 2012;98:523–24. http://dx.doi.org/10.1136/heartjnl-2012-301753
- Haga K, Murray S, Reid J et al. Identifying community-based chronic heart failure patients in the last year of life: a comparison of the Gold Standards Framework Prognostic Indicator Guide and the Seattle Heart Failure Model. Heart 2012;98:579–83. http://dx.doi.org/10.1136/heartjnl-2011-301021
- Sze S, Pellicori P, Zhang J, Weston J, Clark AL. Identification of frailty in chronic heart failure. JACC Heart Fail 2019;7:291–302. https://doi.org/10.1016/j.jchf.2018.11.017 [Epub ahead of print]
- Sze S, Pellicori P, Kazmi S et al. Prevalence and prognostic significance of malnutrition using 3 scoring systems among outpatients with heart failure: a comparison with body mass index. JACC Heart Fail 2018;6:476–86. https://doi.org/10.1016/j.jchf.2018.02.018 [Epub ahead of print]
- Sze S, Pellicori P, Zhang J, Weston J, Squire IB, Clark AL. Effect of frailty on treatment, hospitalisation and death in patients with chronic heart failure. Clin Res Cardiol 2021;110:1249–58. https://doi.org/10.1007/s00392-020-01792-w [Epub ahead of print]
- Sze S, Pellicori P, Zhang J, Clark AL. Malnutrition, congestion and mortality in ambulatory patients with heart failure. Heart 2019;105:297–306. https://doi.org/10.1136/heartjnl-2018-313312 [Epub ahead of print]
- Sze S, Pellicori P, Zhang J, Weston J, Clark AL. The impact of malnutrition on short-term morbidity and mortality in ambulatory patients with heart failure. Am J Clin Nutr 2021;113:695–705. https://doi.org/10.1093/ajcn/nqaa311
- French M, Keegan T, Anestis E, Preston N. Exploring socioeconomic inequities in access to palliative and end-of-life care in the UK: a narrative synthesis [published correction appears in BMC Palliat Care 2021 Dec 12;20:188]. BMC Palliat Care 2021;20:179. https://doi.org/10.1186/s12904-021-00878-0
- Abel AAI, Samuel NA, Cuthbert JJ et al. Hospital admissions in the last year of life of patients with heart failure. Eur Heart J Qual Care Clin Outcomes 2023;10:168–75 https://doi.org/10.1093/ehjqcco/qcad047 [Online ahead of print]
- General Medical Council. Treatment and care towards the end of life: good practice in decision-making. 2022. Available from: http://www.gmc-uk.org/guidance/ethical_guidance/end_of_life_care.asp (accessed 01 November 2023)
- Auerbach S. Should patients have control over their own health care?: Empirical evidence and research issues. Ann Behav Med 2000;22:246–59. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4118537/
- Kelner M. Activists and delegators: elderly patients’ preferences about control at the end of life. Soc Sci Med 1995;41:537–45. https://doi.org/10.1016/0277-9536(94)00381-3
- Flynn KE, Smith MA, Vanness D. A typology of preferences for participation in healthcare decision making. Soc Sci Med 2006;63:1158–69. http://dx.doi.org/10.1016/j.socscimed.2006.03.030
- Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ 2010;340:c1345. http://dx.doi.org/10.1136/bmj.c1345
- Billingham MJ, Billingham SJ. Congruence between preferred and actual place of death according to the presence of malignant or non-malignant disease: a systematic review and meta-analysis. BMJ Support Palliat Care 2013;3:144–54. http://dx.doi.org/10.1136/bmjspcare-2012-000292
- National End of Life Care Intelligence Network. Deaths from cardiovascular diseases: implications for end of life care in England. NHS National End of Life Care Programme 2013. Available from: http://www.endoflifecare-intelligence.org.uk/resources/publications/deaths_from_cardiovascular_diseases (accessed November 2023)
- Beattie JM, Connolly MJ, Ellershaw JE. Deactivating implantable cardioverter defibrillators. Ann Intern Med 2005;143:690–1. https://dx.doi.org/10.3238/arztebl.2012.0535
- Allen LA, Stevenson LW, Grady KL et al. Decision making in advanced heart failure. Circulation 2012;125:1928–52. http://dx.doi.org/10.1161/CIR.0b013e31824f2173
- Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage 2006;31:58–69. https://doi.org/10.1016/j.jpainsymman.2005.06.007
- National Institute of Health and Care Excellence. Improving supportive and palliative care for adults with cancer [CSG4]. London: NICE, 2004. Available from: https://www.nice.org.uk/guidance/csg4 (accessed 30 January 2024)
- Zacharias H, Raw J, Nunn A, Parsons S, Johnson M. Is there a role for subcutaneous furosemide in the community and hospice management of end-stage heart failure? Palliat Med 2011;25:658–63. https://doi.org/10.1177/0269216311399490
- Gilotra NA, Princewill O, Marino B et al. Efficacy of intravenous furosemide versus a novel, pH-neutral furosemide formulation administered subcutaneously in outpatients with worsening heart failure. JACC Heart Fail 2018;6:65–70. https://doi.org/10.1016/j.jchf.2017.10.001 [Epub ahead of print]
- Galbraith S, Fagan P, Perkins P, Lynch A, Booth S. Does the use of a handheld fan improve chronic dyspnea? A randomized, controlled, crossover trial. J Pain Symptom Manage 2010;39:831–8. http://dx.doi.org/10.1016/j.jpainsymman.2009.09.024
- Oxberry S, Bland J, Clark A, Cleland J, Johnson M. Repeat dose opioids may be effective for breathlessness in chronic heart failure if given for long enough. J Palliat Med 2013;16:250–5. http://dx.doi.org/10.1089/jpm.2012.0270
- Johnson MJ, Cockayne S, Currow DC et al. Oral modified-release morphine for breathlessness in chronic heart failure: a randomized placebo-controlled trial. ESC Heart Fail 2019;6:1149–60. https://doi.org/10.1002/ehf2.12498 [Epub ahead of print]
- Simon ST, Higginson IJ, Booth S, Harding R, Bausewein C. Benzodiazepines for the relief of breathlessness in advanced malignant and non-malignant diseases in adults. Cochrane Db Syst Rev 2010;20:CD007354. http://dx.doi.org/10.1002/14651858.CD007354.pub2
- Cranston Josephine M, Crockett A, Currow D. Oxygen therapy for dyspnoea in adults. Cochrane Db Syst Rev 2008;16:CD004769. http://dx.doi.org/10.1002/14651858.CD004769.pub2
- Clark AL, Johnson M, Fairhurst C et al. Does home oxygen therapy (HOT) in addition to standard care reduce disease severity and improve symptoms in people with chronic heart failure? A randomised trial of home oxygen therapy for patients with chronic heart failure. Health Technol Assess 2015;19:1–120. https://dx.doi.org/10.3310/hta19750
- Ong HL, Abdin E, Seow E et al. Prevalence and associative factors of orthostatic hypotension in older adults: results from the well-being of the Singapore elderly (WiSE) study. Arch Gerontol Geriatr 2017;72:146–52. https://doi.org/10.1016/j.archger.2017.06.004
- Finucane C, O’Connell MD, Donoghue O, Richardson K, Savva GM, Kenny RA. Impaired orthostatic blood pressure recovery is associated with unexplained and injurious falls. J Am Geriatr Soc 2017;65:474–82. https://doi.org/10.1111/jgs.14563


All rights reserved. No part of this programme may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publishers, Medinews (Cardiology) Limited.
It shall not, by way of trade or otherwise, be lent, re-sold, hired or otherwise circulated without the publisher’s prior consent.
Medical knowledge is constantly changing. As new information becomes available, changes in treatment, procedures, equipment and the use of drugs becomes necessary. The editors/authors/contributors and the publishers have taken care to ensure that the information given in this text is accurate and up to date. Readers are strongly advised to confirm that the information, especially with regard to drug usage, complies with the latest legislation and standards of practice.
Healthcare professionals should consult up-to-date Prescribing Information and the full Summary of Product Characteristics available from the manufacturers before prescribing any product. Medinews (Cardiology) Limited cannot accept responsibility for any errors in prescribing which may occur.