Background
Major epidemiological studies have shown a direct, consistent relationship between non-high-density lipoprotein cholesterol (non-HDL-C) lowering and coronary heart disease (CHD) risk reduction.1 Non-HDL-C is calculated by subtracting HDL-C from total cholesterol (see module 3). Retention of cholesterol-rich apolipoprotein B-containing lipoproteins (the non-HDL-C fraction, including low-density lipoprotein cholesterol (LDL-C), lipoprotein(a) [Lp(a)] and remnants) in the arterial wall has been described as the root cause for the initiation and progression of atherosclerosis.2
Very high cholesterol levels are seen in inherited disorders, such as familial hypercholesterolaemia (FH) where LDL-C is typically double that of unaffected family members; the likelihood of developing premature and often fatal heart disease is greatly increased. The majority of these who will develop atherosclerosis, however, may have only a moderately elevated LDL-C, which may be considered ‘normal’ for a Western industrialised society. Many populations in the developing world with negligible CHD mortality rates will have LDL-C levels of 2 mmol/L or less, probably reflecting those of our hunter-gatherer ancestors.
Several genetic variants have been identified as being associated with lower serum LDL-C levels and consequently lower lifetime cardiovascular (CV) risk. A Mendelian randomisation study of a selection of these polymorphisms has shown that lifelong exposure to lower levels is linked with a substantially lower risk of CHD.3 Mutations resulting in the inactivation of the LDL receptor (LDLR) regulatory protein, proprotein convertase subtilisin/kexin-9 (PCSK9), have been linked with particularly low LDL-C levels and CV risk and PCSK9 has become a pharmaceutical target.4,5
The evidence base6
Many randomised clinical trials over the past 30 years or so have demonstrated that lowering cholesterol reduces CV events. The Cholesterol Treatment Trialists Collaboration (CTTC) reviewed data from 27 clinical trials of statins (170,000 participants) for both primary and secondary prevention and reported a 22% relative risk reduction per 1 mmol/L reduction in LDL-C concentration for major vascular events. There was no evidence of any threshold within the cholesterol range studied, suggesting that reduction of LDL-C by 2−3 mmol/L would reduce risk by about 40−60%. These beneficial effects were seen in both men and women, at ages from ≤65 to ≥75 years, in people with and without cardiovascular disease (CVD), in those at high and low CV risk, in patients with diabetes mellitus (DM), and in those with average levels of blood cholesterol. No excess in non-CV mortality, including cancers, was observed with lipid lowering.
Treatment targets – how low to go?
Several treatment strategies have been proposed for the management of dyslipidaemia, including ‘treat to target,’ ‘fire and forget’ and ‘the lower the better’.
Primary prevention
The National Institute of Clinical and Health Excellence (NICE) clinical guideline (NG238) recommends a 40% reduction in non-HDL-C from baseline for primary prevention and therefore, atorvastatin 20 mg or higher, rosuvastatin 10 mg or higher, or any combination of atorvastatin and ezetimibe would achieve this reduction.7 Additionally, NICE have specific LDL-C thresholds for the initiation of injectable lipid-lowering therapies, which are outlined later in this module.
The 2019 European Atherosclerosis Society (EAS) recommend much lower LDL-C targets for primary prevention based on the individual’s CV risk (please refer to table 1).8
Secondary prevention
- The 2019 EAS8 and the 2023 European Society of Cardiology (ESC)9 recommend LDL-C levels <1.4 mmol/L as well as ≥50% reduction from baseline as a current treatment goal for secondary prevention
- Both the 2019 ESC/EAS and 2023 ESC guidelines recommend that in very high-risk patients who experience a second vascular event within two years, an LDL-C levels <1 mmol/L should be targeted as levels lower than this have shown added benefits8,9
- NICE 2023 (NG238) recommends an LDL-C treatment target of <2.0 mmol/L or a non-HDL-C <2.6 mmol/L for secondary prevention.7
Evidence from intravascular ultrasound studies of coronary atherosclerosis involving statin-treated patients suggest that disease progression can be substantially arrested – and regression promoted – at achieved LDL-C concentrations ≤1.8 mmol/L (70 mg/dL), but at present, a cost-effectiveness analysis over a long time period is lacking.8,10
|
A recently introduced NHS England Quality and Outcomes Framework (QOF) indicator recommends that patients on the QOF Coronary Heart Disease, Peripheral Arterial Disease, or Stroke/Transient Ischaemic Attack (TIA) Register should have a recording of non-HDL-C <2.5 mmol/L or LDL-C <1.8 mmol/L in the last 12 months.11 |
Please visit BJC TV for more information https://tv.bjcardio.co.uk/cardiovascular-medicine/qof-indicators-for-cvd-prevention/.
The NICE 2023 update to NG238 recommend a secondary prevention treatment targets of LDL-C level <2.0 mmol/L and non-HDL-C level <2.6 mmol/L, which are higher than the 2023 QOF indicator targets, 2019 ESC/EAS guidelines and 2014 Joint British Societies recommendations.7 NICE recognises that their secondary prevention treatment targets are higher than other guidelines and those used in general practice and that in people with ASCVD, LDL-C and non-HDL-C levels should be lowered as much as possible.7
The treatment targets recommended by NICE, however, are based on cost-effectiveness and NICE does not consider it to be cost-effective to offer the full range of lipid-lowering treatments to all patients with ASCVD. Nationally, the differences in NICE and QOF guidance may cause confusion, especially as NICE agrees that ezetimibe should be considered in combination with other lipid-lowering therapy, even in patients who are below the treatment target. Considering the above guidance and in line with HEART UK, in patients with CVD, LDL-C and non-HDL-C should be lowered according to the maximal tolerated dose of lipid-lowering therapy.
Table 1. Recommendations for treatment goals7,8,12
| CV risk category (ESC/EAS)8 | Recommended treatment goal |
Low risk
|
LDL-C <3.0 mmol/L |
Moderate risk
|
LDL-C <2.6 mmol/L |
High risk
|
LDL-C <1.8 mmol/L and ≥50% reduction from baseline |
Very high risk
|
LDL-C <1.4 mmol/L and ≥50% reduction from baseline |
| Patients with ASCVD who experience a second vascular event within two years on maximally tolerated statin therapy | LDL-C <1.0 mmol/L |
| CV risk category (NICE)7 | Recommended treatment goal |
| Primary prevention | 40% reduction in non-HDL-C |
| Secondary prevention | LDL-C <2.0 mmol/L |
| CV risk factor (HEART UK Consensus 2019)12 | Recommended treatment goal |
| Lipoprotein(a) >90 nmol/L | Non-HDL-C <2.5 mmol/L or LDL-C <1.8 mmol/L |
| Key: ASCVD = atherosclerotic cardiovascular disease; BP = blood pressure; CKD = chronic kidney disease; CV = cardiovascular; CVD = cardiovascular disease; DM = diabetes mellitus; EAS = European Atherosclerosis Society; eGFR = estimated glomerular filtration rate; ESC = European Society of Cardiology; FH = familial hypercholesterolaemia; LDL-C = low-density lipoprotein cholesterol; NICE = National Institute for Health and Care Excellence; non-HDL-C = non-high-density lipoprotein cholesterol; SCORE = Systematic Coronary Risk Evaluation; TC = total cholesterol; T1/2DM = type 1 or type 2 diabetes mellitus | |
Lifestyle intervention
Therapeutic lifestyle intervention underpins the management of dyslipidaemia. Indeed, the ESC/EAS guidelines on dyslipidaemia place emphasis on nutritional approaches, either alone or complementary to pharmacotherapy, in managing hypercholesterolaemia to reduce CV risk.8
NICE recommends referring patients to exercise programmes and weight management services. Applicable NICE guidelines are available:
- on behaviour change (https://www.nice.org.uk/guidance/ph49)
- on physical activity (https://www.nice.org.uk/guidance/ph54)
- on lifestyle services for people who are overweight or have obesity (https://www.nice.org.uk/guidance/ph53)
Whilst lifestyle intervention plays a key role in managing dyslipidaemia, in patients living with obesity, the treatment of choice is multimodal intervention, including lifestyle modification, with or without pharmacotherapy, and with or without bariatric surgery. Discussing weight management options in adults who are overweight or have obesity is essential.
Offer adults with a body mass index (BMI) ≥40 kg/m2 or BMI ≥35 kg/m2 with obesity-related comorbidity referral to specialist weight management services for full assessment and consider pharmacotherapy and/or bariatric surgery.
For patients with a BMI of 30–34.9 kg/m2 who have had a recent (within the last 10 years) diagnosis of type 2 DM, consider expedited assessment for bariatric surgery.
BMI cutoffs are reduced by 2.5 kg/m2 in people with Black African, Afro-Caribbean, Middle Eastern, Chinese, South Asian and other Asian family background.13
For more details, see the chapter on lifestyle interventions available at https://www.nice.org.uk/guidance/cg189/chapter/Recommendations#lifestyle-interventions
Diet and alcohol
Dietary intervention favourably influences lipids and CV risk and should therefore be a cornerstone of treatment efforts. The Mediterranean diet still has the strongest evidence for primary and secondary CVD prevention.9,10 Unlike previous evidence suggesting a moderate amount of alcohol could be beneficial, recent data suggest that any amount of alcohol can increase weight and blood pressure, and people who are abstinent from alcohol have the lowest risk of CVD outcomes.9 The ESC 2023 guidance recommends cutting down alcohol intake to a maximum 100 g (12.5 units) per week for both men and women as higher amounts are associated with lower life expectancy.10
Improving diet by substituting saturated fats with unsaturated or monosaturated fats not only lowers LDL-C, but also benefits triglycerides (TGs) and HDL-C. For primary and secondary prevention of CVD, NICE NG238 recommends reducing daily total fat intake to ≤30% of one’s total energy intake, of which ≤7% is saturated fats.7
There is growing support for the value of so-called ‘functional foods’ in the diet. Consumption of plant sterols or stanols is consistently associated with lowering of LDL-C levels by up to 10%.14 Additionally, increasing intake of soluble (viscous) fibre, such as in oats, can produce modest reductions in total and LDL-C (by about 0.13 mmol/L).15 Advice on incorporating such foods into the diet is included in 2019 ESC/EAS guidelines, especially for patients for whom total CV risk assessment does not justify the use of pharmacotherapy.8 Comparison shows that dietary changes can produce a cumulative 20−30% reduction in LDL-C (table 2).16
Table 2. Effects of dietary changes on LDL-C
| Dietary component | Dietary change | Approximate reduction in LDL-C |
| Reducing saturated fat | From 15% to 6% of calories | 11% |
| Reducing dietary cholesterol | <300 mg/day* | 5% |
| Weight reduction | 5% weight loss | 10% |
| Functional foods | ||
| Viscous fibre | 5–10 g/day | 5% |
| Plant sterols/stanols | 2–3 g/day | 6–15% |
| Effect with combined dietary intervention | 20–30% | |
| Key: LDL = low-density lipoprotein; LDL-C = low-density lipoprotein cholesterol * 100 mg/day reduction of cholesterol reduces total cholesterol by 1% Reproduced with kind permission from Viljoen10 |
||
A NICE-endorsed lipid management pathway17 created by the NHS England Accelerated Access Collaborative conveniently brings together all of the NICE guidance, quality standards and materials to support implementation of CVD prevention, including the ‘cardioprotective diet’ and physical exercise. This is available at: https://www.nice.org.uk/search?q=dyslipidaemia
Physical activity
Exercise is also fundamental for improving lipids and reducing CV risk. While physical activity improves LDL-C levels, there is even greater benefit in terms of lowering TGs (by up to 20%) and raising HDL-C levels (by up to 10%). Not all of the cardioprotective effect of exercise is due to exercise-mediated weight loss, as there is evidence that both weight loss and physical exercise act independently and synergistically to improve lipids and CV risk. Sedentary behaviour is an independent risk factor for all-cause mortality and even adults with chronic conditions should be encouraged to reduce their sedentary time and replace it with physical activity, including light-intensity exercise.10
Pharmacological management of dyslipidaemia
Important notice: prescribers should consult the British National Formulary and Summary of Product Characteristics (SPC) for more extensive advice on prescribing and use of all of the lipid-modifying agents discussed in this module.
β-Hydroxy β-methylglutaryl-co-enzyme A (HMG-CoA) reductase inhibitor (statin) treatment remains the cornerstone of lipid-modifying treatment to prevent CVD. Consideration may be given to the need for additional treatment for lowering elevated TGs, in accordance with current guidelines.
In January 2023 following changes in guidance, NICE estimated that on average, for every 1,000 people with a risk of 5% over the next 10 years who take a statin, about 20 people will not get heart disease or have a stroke because they take a statin. This figure doubles to 40 for people with a risk of 10%. For people with a 20% risk, NICE estimates that on average, around 70 people would not get heart disease or have a stroke within the next 10 years.18
In an update to its 2008 guidance on lipid modification,9 NICE recommends that the threshold for starting preventative treatment for CVD should be halved from a 20% risk of developing CVD over 10 years to a 10% risk. Up to 4.5 million people could be eligible for statins under the lower threshold. Offering statins to all eligible people could prevent up to 28,000 heart attacks and 16,000 strokes each year. In 2023, NICE recommends considering statin in primary prevention, even in patients with <10% risk as part of shared decision making.
In the latest NICE clinical guideline NG238, key recommendations include:7
- Identifying CVD risk using the QRISK®3* (instead of QRISK®2) assessment tool for the primary prevention of CVD in people aged 25–84 years, including patients with type 2 DM
- Prioritising people for a full formal risk assessment if their estimated 10-year risk of CVD is 10% or more
- One of the limitations of using a 10-year risk assessment tool is underestimating CVD risk in younger patients with CVD risk factors. Therefore, lifetime risk assessment tools like QRISK®3-lifetime should be used in patients younger than 40 years with a CVD risk factor or patients with 10-year QRISK®3 score of less than 10%
- As part of shared decision making, to consider statin for primary prevention even in people with a 10-year QRISK®3 score of less than 10%
- QRISK®3 is not validated in patients with type 1 DM, chronic kidney disease (CKD) and familial hypercholesterolaemia (FH); these patients are already considered to be at high risk of developing CVD
- People older than 85 years already have an increased risk of CVD, due to their age alone, and should be considered for lipid-lowering treatment
- Before starting lipid-modification therapy, arrange a baseline full lipid profile (fasting is not needed), and exclude secondary causes of dyslipidaemia
- A full lipid profile and liver transaminases should be repeated two to three months after starting treatment or making any changes
- For secondary prevention, the new treatment target is LDL-C ≤2 mmol/L or non-HDL-C ≤2.6 mmol/L. The recommendation for primary prevention remained the same with achievement of >40% reduction in non-HDL-C from baseline considered satisfactory
- A full lipid profile should be requested on admission to hospital with acute coronary syndrome (ACS) and lipid-lowering treatment started/escalated as early as possible
- A repeat full lipid profile is necessary after two to three months to check for side effects and to guide as to whether treatment intensification is required.
NICE notes that in primary prevention, not everyone with a 10% or greater risk of CVD within 10 years will need to take a statin immediately and advises that preventative lifestyle measures are adopted first. For secondary prevention, however, statin treatment should not be delayed and lifestyle optimisation should be discussed along with starting treatment.
* QRISK®3 (https://www.qrisk.org/three/), described in module 2, is an updated version of the QRISK®2 calculator, which considers additional risk factors, including CKD stage 3 or above, migraine, corticosteroid use, systemic lupus erythematosus (SLE), use of atypical antipsychotics, severe mental illness, erectile dysfunction and a variability of systolic blood pressure.
Be aware that CVD risk assessment tools may underestimate risk in some group of patients, including those with autoimmune disorders or another systemic inflammatory disease, patients with severe mental illness (bipolar disorder, schizophrenia and other psychoses), patients on immunosuppressant medications, or those treated for HIV.
The summary of lipid management for primary and secondary CVD prevention can be found here: https://www.england.nhs.uk/aac/wp-content/uploads/sites/50/2020/04/lipid-management-pathway-v6.pdf
Statins (HMG-CoA reductase inhibitors)
Five statins are currently available in the UK: simvastatin, pravastatin, fluvastatin, atorvastatin and rosuvastatin. Statins are grouped into three different intensity categories according to the percentage reduction in LDL-C they produce (see table 3):
- ‘low intensity’ if the reduction is 20% to 40%
- ‘medium intensity’ if the reduction is 31% to 40%
- ‘high intensity’ if the reduction is above 40%.
Table 3. The grouping of statins into intensity category
| Statin | Percentage reduction in LDL-C (%) | |||||
| Dose (mg/day) | 5 | 10 | 20 | 40 | 80 | |
| Fluvastatin | – | – | 21 | 27 | 33 | |
| Pravastatin | – | 20 | 24 | 29 | – | |
| Simvastatin | – | 27 | 32 | 37 | 42* | |
| Atorvastatin | – | 37 | 43 | 49 | 55 | |
| Rosuvastatin | 38 | 43 | 48 | 53 | – | |
| Key: LDL-C = low-density lipoprotein cholesterol | ||||||
| Low intensity | 20–30% | * Advice from the Medicines and Healthcare products Regulatory Agency: there is an increased risk of myopathy associated with high-dose (80 mg) simvastatin. The 80 mg dose should be considered only in patients with severe hypercholesterolaemia and high risk of CV complications who have not achieved their treatment goals on lower doses, when the benefits are expected to outweigh the potential risks. | ||||
| Medium intensity | 31–40% | |||||
| High intensity | >40% | |||||
| Data from NICE NG2387 | ||||||
Statins were once thought to have non-lipid pleiotropic effects, including anti-inflammatory activity, and the ability to improve endothelial dysfunction and plaque stability. To what extent these actions contribute to the beneficial effect of statins is not yet established, but the benefits of statins are most clearly related to the achieved reduction of LDL-C, non-HDL-C and apolipoprotein B levels.
Indications
Statins are indicated for the reduction of elevated total cholesterol and LDL-C in adult patients with primary hypercholesterolaemia (heterozygous familial and non-familial) and mixed dyslipidaemia when response to diet and other non-pharmacological measures are inadequate.
Mode of action
Statins work by inhibiting the mevalonate pathway and thus hepatic cholesterol synthesis (figures 1 and 2) and upregulate hepatic LDLR activity.
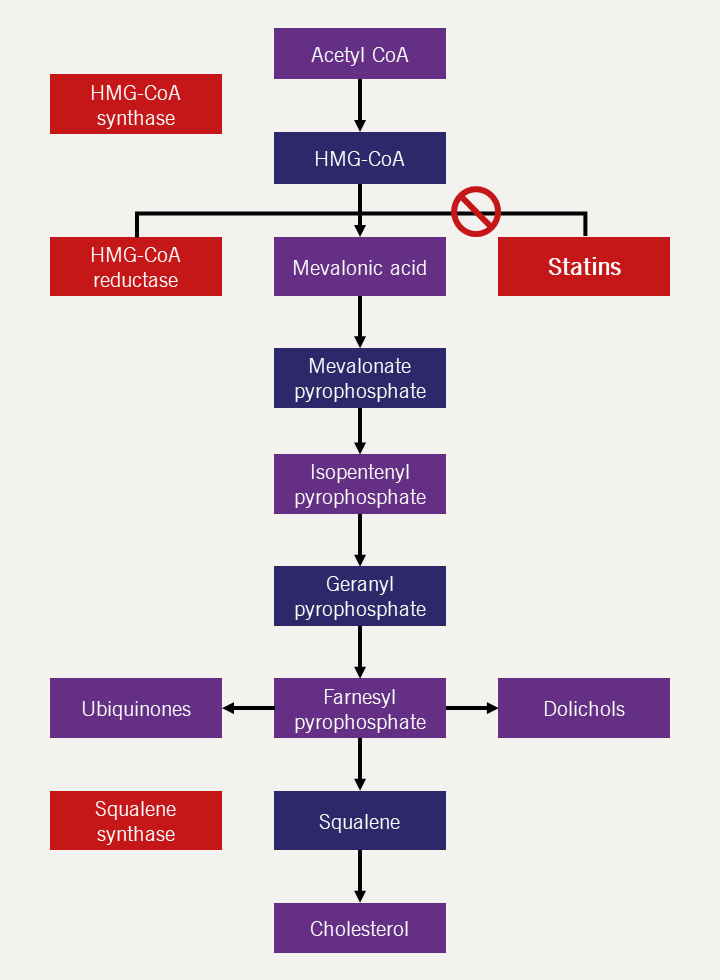
| Key: CoA = co-enzyme A; HMG-CoA = β-Hydroxy β-methylglutaryl-co-enzyme A |
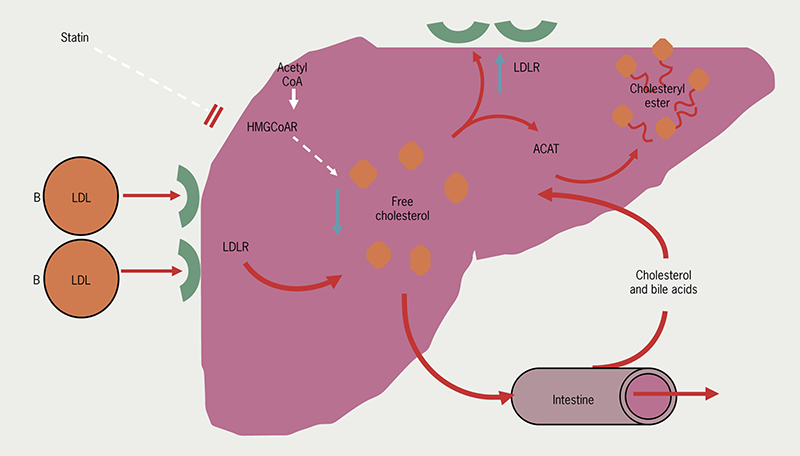
| Key: ACAT = Acyl-CoA-cholesterol acyltransferase; HMG-CoAR = HMG-Co A reductase; LDL = low-density lipoprotein; LDLR = low-density lipoprotein receptor |
Efficacy
- Up to 55% reduction in LDL-C (see table 3)
- Modest fall in TGs (10–30%).
Advantages
Over the past five years, more evidence is available on the benefits and side effects of statins. This was reflected in the 2023 guidelines where NICE highlighted the benefits of statins and emphasised that the incidence of severe muscle adverse effects is extremely low. The advantages of statins are that they are:
- Potent
- Mostly well tolerated
- Only require a single, nightly dose.
Adverse effects
|
Statin intolerance is defined as the presence of clinically significant adverse effects from statin therapy that are considered to represent an unacceptable risk to the patient or that may result in adherence to therapy being compromised. |
To guide clinicians, a statin intolerance pathway has been developed by the Accelerated Access Collaborative (AAC), which has been endorsed by NICE. It is available on the NHS England AAC page here.
- Muscle-related adverse effects:
Statins are generally well tolerated and serious adverse events are rare. The most serious side effect is myopathy, which can progress to rhabdomyolysis; however, the incidence of myopathy is low (less than one per 1,000 treated patients).8 Creatine kinase (CK) is the primary marker of damage. Myopathy is more likely to occur in patients with complex medical problems or on multiple medications and in the elderly. Myalgia without CK elevation occurs in 5–10% of patients, many of whom can continue the medication if the symptoms are tolerable. It is suggested that the risk of muscle symptoms is lower with hydrophilic statins, such as pravastatin and rosuvastatin. - Liver-related adverse effects:
Although statin therapy can lead to temporary increases in concentrations of liver enzymes, it is associated with very low rates of serious liver injury (about one case per 100,000 users).19 Alanine aminotransferase (ALT) is a marker of hepatocellular damage and can raised in 0.5–2.0% of statin-treated patients and is dose dependent. Treatment should be discontinued if there is an increase in ALT more than three times the upper limit of normal. Progression to liver failure is exceedingly rare but levels should be monitored, and high levels may resolve with dose reduction. - Hyperglycaemia:
The small risk is outweighed by the CV benefits.
|
Editors’ note: |
Metabolism and drug–drug interactions
Many statins undergo significant hepatic metabolism via cytochrome P450 enzymes (CYPs), except pravastatin and rosuvastatin. Therefore, there is the potential for interaction with other drugs, such as warfarin, which are metabolised through the same pathway.
Patients prescribed simvastatin or atorvastatin should be advised to avoid consuming grapefruit products whilst on these medications. Although the studies concerning grapefruit interactions with pravastatin, fluvastatin, or rosuvastatin are not as significant, it is probably advisable not to consume grapefruit juice a few hours before or after taking this medication and to be moderate in the consumption of (or avoid) grapefruit-containing products.
Combinations of statins and fibrates may enhance the risk of myopathy; this is highest for gemfibrozil and gemfibrozil should not be used in combination with a statin, but the increased risk of myopathy is small when statins are combined with other fibrates e.g. fenofibrate, bezafibrate. Some of the drugs that statins may interact with are shown below:
- fibrates (especially gemfibrozil)
- ciclosporin
- tacrolimus
- macrolide antibiotics (quinolones)
- warfarin
- digoxin
- calcium channel blockers, especially verapamil, possibly diltiazem, dihydropyridines
- azole antifungals
- antiretrovirals
- amiodarone.
Cholesterol absorption inhibitors (ezetimibe)
There is only one drug currently available in this class – ezetimibe. This has been available for over a decade and is usually combined with a statin to achieve additional reductions in LDL-C levels. IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) showed that starting ezetimibe with/in addition to a statin within 10 days of an ACS is safe and has long-term CV outcome benefits compared to statin monotherapy.20 Therefore, in patients who are admitted with an ACS and are already on a maximum-tolerated dose of a statin, or who have LDL-C levels which are unlikely to reach target goal with statin treatment alone, intensifying lipid-lowering treatment with ezetimibe should be considered as early as possible.21 NICE has published technology appraisal guidance (TA385) for ezetimibe in primary hypercholesterolaemia.22
Indications
Ezetimibe is indicated as an add-on to dietary measures to:
- reduce LDL-C levels in people with primary hyperlipidaemia, alone or with a statin
- reduce LDL-C levels in people with mixed hyperlipidaemia, in combination with fenofibrate
- reduce LDL-C levels in people with homozygous familial hypercholesterolemia (HoFH), in combination with specific statins
- reduce levels of circulating phytosterols in people with homozygous sitosterolaemia (a rare inherited disorder).
Mode of action
This acts as a specific inhibitor of cholesterol absorption in the small bowel (figure 3), without affecting the absorption of fat-soluble nutrients. Ezetimibe blocks the enterohepatic recirculation of cholesterol by inhibiting NPC1L1 (Niemann-Pick C1-like 1) transporter. This indirectly leads to a reduction of hepatic cholesterol and upregulation of LDLRs, mimicking the action of statins.
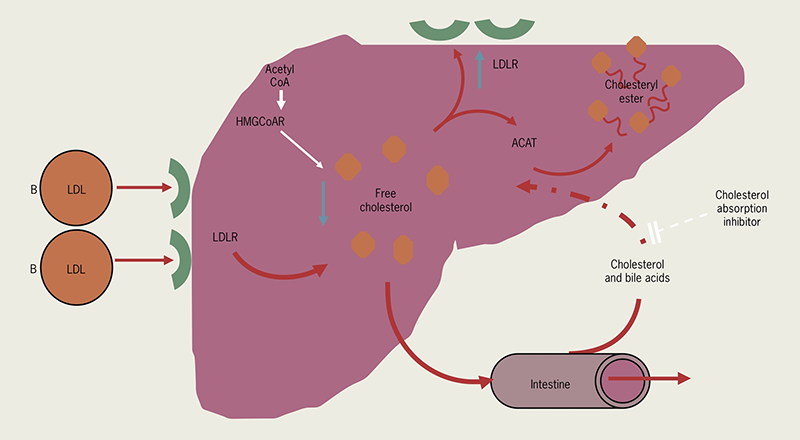
| Key: ACAT = Acyl-CoA-cholesterol acyltransferase; HMG-CoAR = HMG-Co A reductase; LDL = low-density lipoprotein; LDLR = low-density lipoprotein receptor |
Efficacy
- Up to 15–20% reduction in LDL-C
- Modest fall in TGs (10–20%).
Advantages
- Mostly well tolerated
- Single, nightly dose.
Stanol ester and sterol ester margarines (see above) have a weaker inhibitory effect on the cholesterol absorption effect through an unrelated mechanism. Typical LDL-C reduction is less than 10%.
Side effects and interactions
There is little evidence of any hepatotoxicity associated with ezetimibe. No major side effects have been reported. The most frequently observed side effects are:
- moderate elevation of liver enzymes
- muscle pain and muscle toxicity (rare)
- gastrointestinal upset.
No dosage adjustment is needed in patients with mild hepatic impairment or mild to severe renal insufficiency.
PCSK9 inhibitors
Targeting the PCSK9 pathway is a mechanism for lowering LDL-C levels. Two PCSK9 inhibitors approved for use by NICE in June 2016 are the monoclonal antibodies alirocumab and evolocumab. Monoclonal antibodies are designed to bind to a specific target, while avoiding other targets.
With 40–70% reduction in LDL-C levels, PCSK9 inhibitors are extremely effective and provide long-term CV outcomes benefits. PCSK9 inhibitors are considered safe in hospitalised patients with ACS.10 Recent data has shown that PCSK9 inhibitors can lead to plaque regression and improvement in plaque phenotype in patients admitted to hospital with ACS.23,24
Given the importance of LDL-C reduction early after ACS and the effectiveness of PCSK9 inhibitors, ESC 2023 guidance recommends starting PCSK9 inhibitor treatment during hospital admission in patients with ACS who are not at their LDL-C goal, despite maximum-tolerated lipid-lowering treatment prior to admission.9
Indications
To optimise lipid management in patients at high risk of CVD who have uncontrolled, severe hypercholesterolaemia or mixed dyslipidaemia, despite maximum-tolerated lipid-lowering therapy, NICE has published technology appraisal guidance for LDL-C treatment thresholds for both alirocumab (TA393)25 and evolocumab (TA394).26 Separate thresholds are assigned to non-FH patients with high and very high risk of CVD and FH patients with and without CVD.
Mode of action
PCSK9 normally binds to LDLRs, preventing them from recycling to the surface of hepatocytes and targets them for destruction in the lysosome. By preventing the PCSK9 binding with the LDLR, PCSK9 inhibitors increase the recycling of LDLRs to the cell surface. The resulting increase in the number of LDLRs on hepatocytes facilitates LDL-C clearance from the blood, ultimately leading to LDL-C reduction.
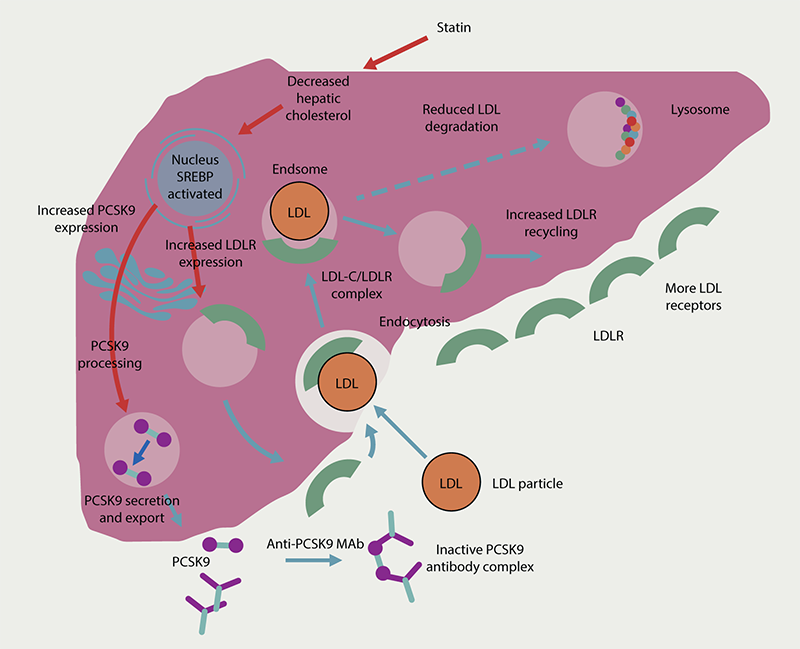
| Key: LDL = low-density lipoprotein; LDL-C = low-density lipoprotein cholesterol; LDLR = low-density lipoprotein receptor; Mab = monoclonal antibody; PCSK9 = proprotein convertase subtilisin/kexin type 9; SREBP – sterol-regulatory-element-binding-protein Adapted from Latimer J, Batty JA, Neely RDG, Kunadian V. PCSK9 inhibitors in the prevention of cardiovascular disease. J Thromb Thrombolysis 2016;42:405–19 https://doi.org/10.1007/s11239-016-1364-1 Work licensed under Creative Commons Attribution 4.0 International license http://creativecommons.org/license/by/4.0 |
Efficacy
- Reductions in LDL-C levels of up to 70% may be achieved with PCSK9 inhibition, independent of background statin therapy5
- Early data suggests a reduction in CV events
Advantages
- Additional LDL-C-lowering capacity in patients intolerant of statins
- Some patients with HoFH may achieve a sufficient LDL-C-lowering effect with the addition of PCSK9 inhibitor treatment in that they may require less frequent lipoprotein apheresis
- Well tolerated
- One subcutaneous injection every two weeks.
Disadvantages
- Lifelong self-administered subcutaneous injections may be unacceptable to some patients
- The increased risk of progression to DM seen with high-intensity statin treatment might also occur with PCSK9 inhibition5
- Short shelf-life
- High cost.
Side effects and interactions
- Very few side effects in clinical studies
- Flu-like symptoms, such as cold, nausea, back and joint pain
- Soreness or itchiness at injection site
- Muscle pain.
Inclisiran
Inclisiran is the first drug in its class indicated for use in adult patients with CVD. It is a small interfering RNA (siRNA) molecule, which reduces LDL-C by inhibiting the synthesis of PCSK9.
Indications
It is indicated in adults with atherosclerotic CVD or heterozygous FH (HeFH) who have elevated LDL-C levels, despite maximum-tolerated therapy.
In 2021, in technology appraisal guidance, NICE recommended inclisiran for the management of primary hypercholesterolaemia or mixed dyslipidaemia only in patients with history of CVD and persistently elevated LDL-C levels ≥2.6 mmol/L, despite maximum-tolerated lipid-lowering treatment.27 The role of inclisiran in primary prevention is being assessed in ongoing clinical trials (VictORION-1 PREVENT [Study of Inclisiran to Prevent Cardiovascular Events in High-Risk Primary Prevention Patients]).
Mode of action
PCSK9 is an enzyme predominantly synthesised in the liver, which promotes lysosomal degradation of the LDLR. Inhibition of this enzyme reduces this degradation, which promotes recycling of the LDLR, increasing hepatic LDLR numbers and therefore increased LDL-C uptake from the circulation. Post-translationally, this mechanism is the target for the monoclonal antibody medications evolocumab and alirocumab, as discussed above. However, inclisiran targets this pathway before translation of PCSK9. Inclisiran is a siRNA, which works through ‘gene-silencing’ pathways to inhibit translation and hepatic production of the PCSK9 enzyme (figure 5).28
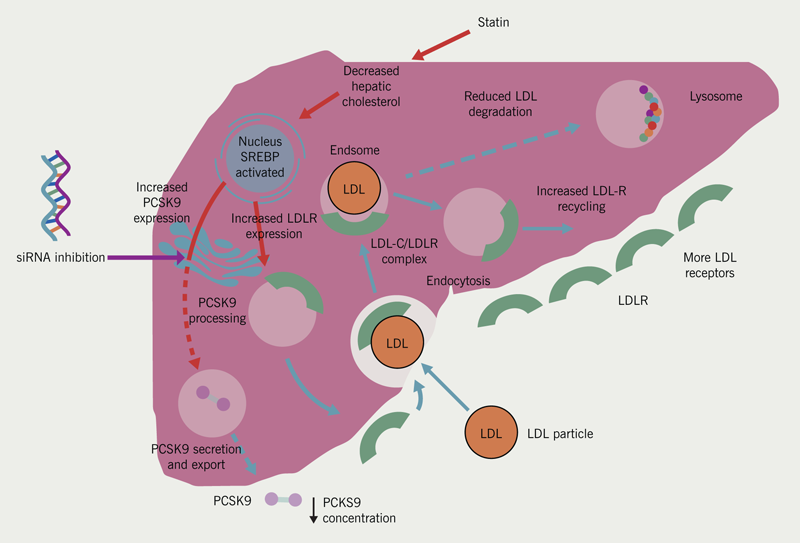
| Inclisiran is a long acting synthetic small interfering RNA (siRNA) which selectively inhibits or ‘silences’ expression of the PCSK9 gene. Unlike anti-PCSK9 monoclonal antibodies, which work at the plasma level, inclisiran acts at an intracellular level within the hepatocytes. The molecule consists of two complementary guide and passenger RNA strands. the guide strand binds to a multiprotein coplex called RNA-induced silencing coplex (RISC) and undergoes hybridisation with complementary mRNA for PCSK9 and induces its degradation. Degradation of mRNA for PCSK9 reduces its translation resulting in decreased PCSK9 protein synthesis and export into the circulation. Consequently there is reduced lysosomal degradation of the LDL receptor, which promotes its recycling resulting in increased hepatic LDL receptor number and increase LDL cholesterol uptake from the circulation. The silencing complex remains active after mRNA degradation so a single siRNA molecule can interfere with the expression of multiple PCSK9 mRNA molecules and has a longer duration of action than anti-PCSK9 monoclonal antibodies. Key: LDL = low-density lipoprotein; LDLR = low-density lipoprotein receptor; PCSK9 = proprotein convertase subtilisin/kexin type 9; siRNA = small interfering RNA; SREBP = sterol-regulatory-element-binding-protein Adapted from Latimer J, Batty JA, Neely RDG, Kunadian V. PCSK9 inhibitors in the prevention of cardiovascular disease. J Thromb Thrombolysis 2016;42:405–19 https://doi.org/10.1007/s11239-016-1364-1 Work licensed under Creative Commons Attribution 4.0 International license http://creativecommons.org/license/by/4.0 |
Efficacy
ORION-8 (Trial to Assess the Effect of Long Term Dosing of Inclisiran in Subjects with High Cardiovascular Risk and Elevated Low-density Lipoprotein Cholesterol), the largest clinical trial to date for inclisiran pooling data from ORION-9 (Trial to Evaluate the Effect of Inclisiran Treatment on Low Density Lipoprotein Cholesterol in Subjects with Heterozygous Familial Hypercholesterolemia), -10 (Inclisiran for Participants With Atherosclerotic Cardiovascular Disease and Elevated Low-density Lipoprotein Cholesterol) and -11 (Inclisiran for Subjects with ASCVD or ASCVD-Risk Equivalents and Elevated Low-density Lipoprotein Cholesterol), showed a mean reduction of 50% in LDL-C levels compared to placebo, which was maintained long term.29 A pooled data analysis of phase 3 clinical trials with 18 months of follow up showed early insights into the potential CV benefits of lowering LDL-C levels with inclisiran,30 but these findings need confirmation with dedicated outcome trials, which are currently running (ORION-4 [A Randomised Trial Assessing the Effects of Inclisiran on Clinical Outcomes Among People with Cardiovascular Disease]).
Advantages
Inclisiran is a long-acting drug, which enables a convenient twice-yearly dosing schedule – this will increase the likelihood of treatment uptake and adherence over medications that need to be taken daily. It is also a further treatment option for patients who are intolerant of statins or are on maximally tolerated treatment who are not reaching LDL-C reduction targets, and who may not be eligible for PCSK9 inhibitors but require further LDL-C lowering.
Disadvantages
- Subcutaneous injections may be unacceptable to some patients.
Side effects and drug–drug interactions
Inclisiran is well tolerated and has shown good safety profiles in clinical trials. Recent data from the ORION-8 trial, which was presented at the ESC 2023 Congress, showed a good long-term safety profile of more than six years in patients with high CV risk and raised LDL-C levels.28 The most common side effects are limited to nasopharyngitis (ORION-3 extension trial [An Extension Trial of Inclisiran in Participants with Cardiovascular Disease and High Cholesterol]) and injection site reactions.7,29,31
Bile acid sequestrants
Bile acid sequestrants were the mainstay of cholesterol-lowering therapy in the pre-statin era. This therapeutic class includes colesevelam, colestipol and the oldest agent, cholestyramine.
Indications
This class of drugs can be used in patients with hypercholesterolaemia as an adjunct to diet (either alone or with a statin), in hyperlipidaemias unresponsive to diet or other measures, and where systemic exposure to statins must be avoided (e.g. pregnancy, early childhood and in patients with a history of serious statin toxicity).
Mode of action
Bile acid sequestrants are non-absorbable anion-exchange resins that bind to bile salts in the gut, preventing their reabsorption from the terminal ileum (figure 6). The resulting depletion in the bile acid pool leads to diversion of cholesterol to form new bile acids. This, in turn, leads to upregulation of LDLRs to maintain the cholesterol pool within the liver and lowering LDL-C levels.
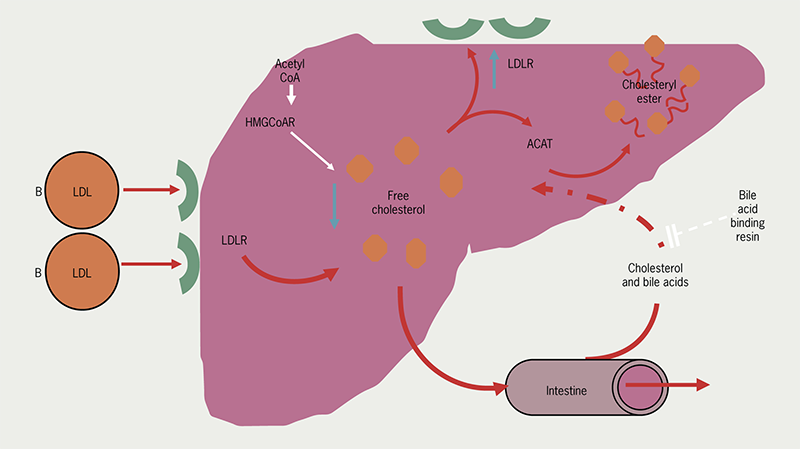
| Key: ACAT = Acyl-CoA-cholesterol acyltransferase; HMG-CoAR = HMG-Co A reductase; LDL = low-density lipoprotein; LDLR = low-density lipoprotein receptor |
Efficacy
- 18–25% LDL-C lowering.
Advantages
- Can reduce glucose levels in hyperglycaemic patients.
Disadvantages
- Can aggravate hypertriglyceridaemia
- May cause folic acid deficiency with long-term use
- Older agents are less palatable.
Side effects and drug–drug interactions
Gastrointestinal side effects, such as constipation or nausea, predominate. Colesevelam is generally better tolerated.
Bile acid sequestrants have important interactions with many commonly prescribed drugs and it is recommended that they are taken either four hours before or one hour after other drugs. Colesevalam is less interactive and can be co-administered with a statin.
Fibrates
Fibric acid derivatives (fibrates) include bezafibrate, ciprofibrate, fenofibrate and gemfibrozil. They are not recommended for isolated hypercholesterolaemia, but they are the drugs of choice when TGs are severely raised (>10 mmol/L) and the risk of acute pancreatitis is the most immediate concern. They may also be of great value in combination with statins in severe mixed hyperlipidaemias (especially familial dysbetalipoproteinaemia or type III dyslipidaemia). They may improve the lipid profiles of patients who have a pattern of moderately high TGs and low HDL-C levels – the most frequent form of dyslipidaemia seen in metabolic syndrome and type 2 DM. In the absence of large-scale trials showing improvement in outcomes, the evidence favours statins as first-line therapy for CV risk reduction.
Indications
Fibrates are indicated in the treatment of moderate to severe hypertriglyceridaemia, in mixed hyperlipidaemia (where the predominant component is raised TG) and in type III dyslipidaemia (usually resulting from an inherited defect of apolipoprotein E).
Efficacy
- Fibrates can lower TGs by up to 50%
- They lower LDL-C by up to 25%
- They raise HDL-C by 10–15%.
Advantages
- Well suited to management of severe hypertriglyceridaemia.
Disadvantages
- Variable effects on LDL-C
- Increased risk of muscle effects if TGs are raised and they are combined with a statin (see Statins above)
- Fibrates are contraindicated in the presence of gallstones/gall bladder disease (increased lithogenicity of bile and risk of gallstones).
Mode of action
The mechanism of action of fibrates involves activation of peroxisome proliferator-activated receptors (PPAR), particularly PPAR-α, leading to reduced hepatic TG synthesis; reduction of apolipoprotein CIII, an endogenous inhibitor of lipoprotein lipase; increased lipoprotein lipase activity (figure 7) and hence increased very-low-density lipoprotein (VLDL) and remnant particle clearance and increased levels of HDL-C.
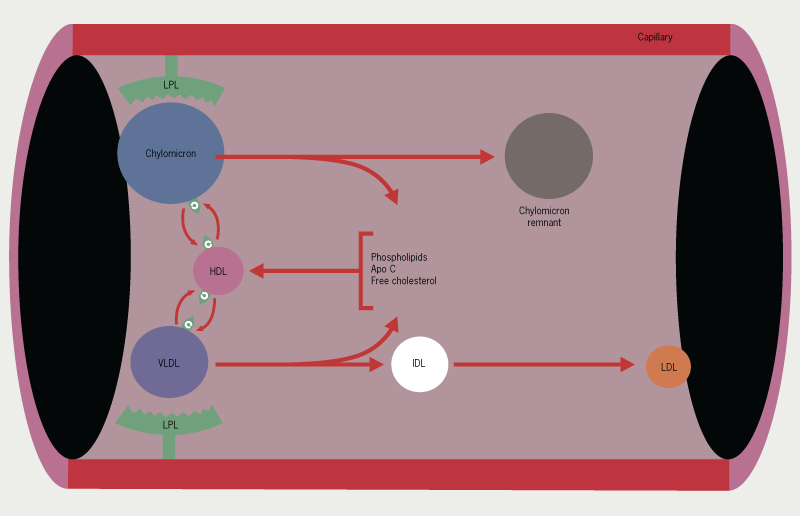
| Key: Apo C = apolipoprotein C; HDL = high-density lipoprotein; IDL = intermediate-density lipoprotein; LPL = lipoprotein lipase; VLDL = very low-density lipoprotein |
Side effects and drug–drug interactions
Side effects with fibrates are generally mild. Gastrointestinal problems are reported in about 5% of patients and skin rashes in 2%. The principle problem is dyspepsia. They may also increase gallstone formation and so should not be used in patients known to have gallstones. Statins appear to reduce the risk of gallstones and the combination may be safer in this respect. Used alone, they may cause myositis and liver enzyme elevations. Gemfibrozil and statins should not be used concomitantly due to the increased frequency of severe myopathy.
Nicotinic acid (niacin)
Nicotinic acid is related to nicotinamide, part of the vitamin B group. In the past it has been considered for use as an adjunct to a statin or where a statin was not tolerated.
It is no longer used as a prominent side effect is prostaglandin-mediated cutaneous vasodilatation, which often leads to profound facial flushing. It was considered that it may reduce long-term CV events but the most widely prescribed formulation of niacin was withdrawn from the European market for commercial reasons. Similarly, a preparation of niacin combined with an ‘anti-flushing’ (anti-prostaglandin) agent – laropiprant – was withdrawn throughout the European Union in 2013 after a study (HPS2-THRIVE: Treatment of HDL to Reduce the Incidence of Vascular Events) showed a failure to reduce major vascular events and an increase in non-fatal serious events.32
Fish oils (omega-3 fatty acids)

| https://commons.wikimedia.org/wiki/File:Inuit_fishing_for_sheefish_at_Selawik_NWR.jpg |
Populations who consume diets high in marine oil (omega-3 fatty acids), such as the Inuit people of Greenland (see figure 8), have low heart disease rates. These compounds may be used to reduce TGs as an alternative or in addition to fibrates in patients with combined (mixed) hyperlipidaemia not controlled by a statin alone. Except for icosapent ethyl (see below), there is little evidence that the TG-lowering effects of fish oils decreases CV risk and NICE does not currently recommend using omega-3 fatty acids, except icosapent ethyl, for the primary or secondary prevention of CVD. It is thought that omega-3 fatty acids reduce plasma TGs levels by increasing fatty acid oxidation, which suppresses hepatic lipogenesis and subsequent VLDL production.33
Icosapent ethyl
Icosapent ethyl, is an ethyl ester of the omega-3 fatty acid, eicosapentaenoic acid (EPA).
Indications
Icosapent ethyl can be used for secondary prevention in patients with elevated TG levels.
In 2022, NICE published technology appraisal guidance (TA805) for icosapent ethyl with statin in people at high risk of CVD with raised TG levels >1.7 mmol/L34 to reduce the risk of CV events. It is only indicated in patients with established CVD and an LDL-C levels between 1.04 mmol/L to 2.6 mmol/L as clinical trial evidence only included patients with LDL-C levels within this range.
Mode of action
The exact mechanisms in which icosapent ethyl leads to lower rates of CV events is not known. Its actions are likely multifactorial and include reduction in TG-rich particles, as well anti-inflammatory, antioxidant and antiplatelet effects.
Efficacy
Results of REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl – Intervention Trial), involving more than 8,000 patients with either established CVD or at high risk of developing CVD and moderately elevated TG (≥1.53 mmol/L and <5.64 mmol/L), showed that icosapent ethyl significantly reduced the first occurrence of the primary composite end point of CV death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularisation, or hospitalisation for unstable angina (25% relative risk reduction, 4.8% absolute risk reduction, number needed to treat of 21).35
Advantages
- Prior to icosapent ethyl, no treatment was available to reduce the risk of CV events in patients with a high risk of CVD whose LDL-C was well controlled with statin therapy but had elevated TG levels.
Disadvantages
- Twice-daily dosing of two large capsules (25 × 10 mm) is required.
Side effects and drug–drug interactions
The following adverse effects may occur in people taking icosapent ethyl:35
- Bleeding: The most frequent adverse event associated with icosapent ethyl is bleeding.34 Serious bleeding events were more frequently seen in patients who were simultaneously taking antithrombotic agents. Therefore, patients taking anticoagulants or antiplatelets should be monitored.
- Arrythmias: Another important adverse event is atrial fibrillation (AF) or atrial flutter, which was reported in 5.8% of patients compared to 4.5% in placebo group,36 some of which required hospital admission.
- Peripheral oedema
- Constipation
- Musculoskeletal pain
- Gout
- Rash.
|
When starting this treatment, patients should be monitored for clinical symptoms and signs of AF/atrial flutter and an ECG should be performed when required. |
Icosapent ethyl is from fish oil; therefore, patients with known allergy to fish/shellfish are at an increased risk of allergic reactions.
Bempedoic acid
A small-molecule compound, bempedoic acid, is the first drug in its class with a novel mechanism of action. It is administered as a prodrug and is taken orally once daily to lower LDL-C. This drug works through the same pathway as statins and increases LDLR-mediated clearance of LDL-C but inhibits an enzyme two steps upstream from HMG-CoA reductase – the target of statins.37
Indications
Bempedoic acid is indicated on its own or combined with other lipid-lowering medications in patients who are intolerant to statins.
NICE published technology appraisal guidance (TA694) for bempedoic acid with ezetimibe in patients with primary hypercholesterolaemia or mixed dyslipidaemia.38 It is indicated when:38
- Statins are not tolerated or contraindicated
- Ezetimibe is not adequately reducing LDL-C levels.
Mode of action
Bempedoic acid lowers LDL-C levels by inhibition of cholesterol synthesis in the liver, which causes subsequent upregulation of the hepatic LDLRs so that more LDL-C is removed from the circulation. The primary mode of action is to inhibit ATP citrate lyase (ACL), which is an enzyme in the cholesterol biosynthesis pathway upstream of HMG-CoA reductase – the target of statins (figure 9).
Bempedoic acid is administered as a prodrug and is only converted to the active metabolite in the liver and not muscles.39 Theoretically, this will reduce the myotoxicity associated with statins and which represents one of the largest barriers to statin uptake and adherence.
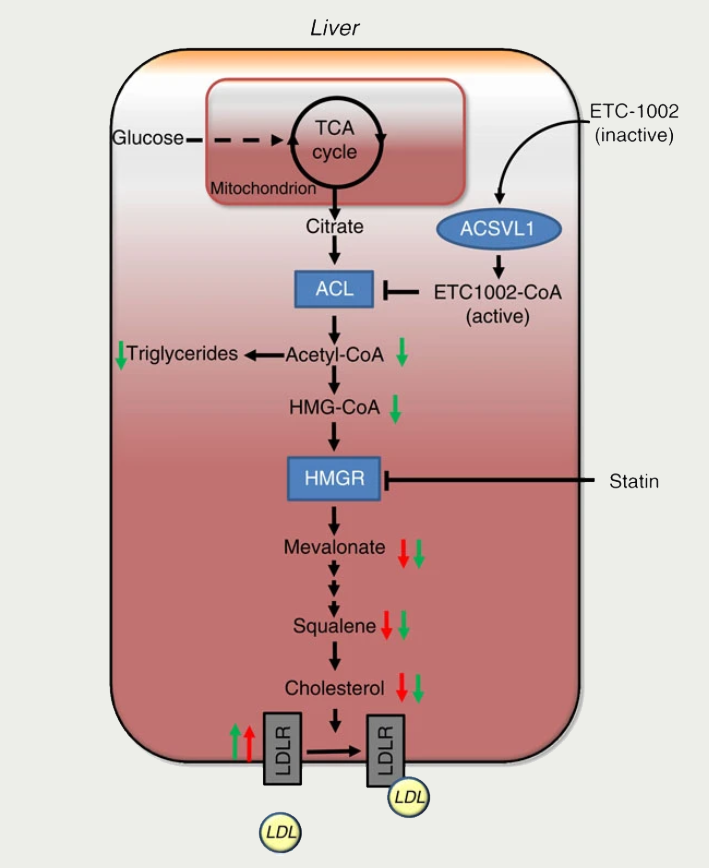
| Key: ACL = ATP-citrate-lyase; ACSVL1 = very long-chain acyl-CoA synthetase-1; HMGR = HMG-CoA reductase; LDL = low-density lipoprotein; LDLR = LDL receptor; TCA = Krebs cycle (tricarboxylic acid cycle) In the liver, ETC-1002 (bempedoic acid) is activated to ETC-1002-CoA by ACSVL1, and subsequently inhibits ACL. Similar to inhibition of HMG-CoA reductase (HMGR) by statins, inhibition of liver ACL by ETC-1002 results in the supression of cholesterol synthesis and compensatory LDLR upregulation and LDL particle clearance from the blood. Green and red arrows indicate the effects of ETC-1002 and statins, respectively. Reproduced with permission from: Pinkosky S, Newton R, Day E et al. Liver-specific ATP-itrate lyase inhibition by bempedoic accid decreases LDL-C and attenuates atherosclerosis. Nat Commun 1016;7:13457. https://doi.org/10.1038/ncomms13457. Work licensed under Createive Commons Attribution 4.0 International Licence http://creativecommons.org/licenses/by/4.0/ |
Efficacy
An analysis of four randomised controlled trials found that bempedoic acid decreased LDL-C by 18% versus placebo when added to maximally tolerated statins in patients with ASCVD, HeFH or both.37
More importantly, a recent double-blinded randomised clinical trial in patients who were statin intolerant showed treatment with bempedoic acid was associated with significantly lower rate of major adverse CV events (MACE) compared to placebo. At six months, bempedoic acid reduced LDL-C by 21.1% in the treatment arm compared to placebo.39
Advantages
The NICE eligibility thresholds for treatment with PCSK9 inhibitors mean many patients remain at LDL-C levels much higher than desirable after maximum tolerated lipid-lowering treatment. In patients not eligible for PCSK9 inhibition or those with statin intolerance, bempedoic acid may be a suitable alternative due to the following characteristics:
- It is an oral formulation
- Single-dosage strength
- It can be combined with ezetimibe into a single pill
- No titration needed
- It carries a low risk of muscle-related adverse effects.
Disadvantages
- It increases plasma concentrations of statins requiring monitoring of patients for statin-related side effects if on both agents
- It may raise serum uric acid and precipitate gout
- It may cause elevation of liver enzymes.
Side effects and drug–drug interactions
Bempedoic acid showed good safety profiles in clinical trials beyond three years of follow up. Compared to placebo, the incidence of gout and cholelithiasis was higher in patients taking bempedoic acid.39
Other side effects include:37,39
- Tendon rupture
- Tendonitis
- Muscle spasms
- Anaemia
- Elevated liver enzymes
- Hyperuricaemia
- Small increase in creatinine.
Tips for prescribers:
- Bempedoic acid inhibits renal tubular OAT2 causing a risk of hyperuricaemia – monitor for signs of gout and treat with urate-lowering drugs as appropriate
- There is a risk of myopathy with concomitant use of bempedoic acid with simvastatin or pravastatin – consider dose reduction of these statins and avoid concomitant use of simvastatin >20 mg or pravastatin >40 mg.
Check out the BJC Lipids Masterclass entitled, ‘Lipids – the good, the bad and the ugly’, where Dermot Neely and Paul Hamilton discuss the different biological lipid transport pathways, how we measure lipids in the blood and how to distinguish between beneficial and non-beneficial lipids, and the national guidelines for lipid management in primary and secondary prevention of CVD.
Treatment summary
A summary of the drugs used in the pharmacological management of dyslipidaemia is shown in table 4.
Table 4. Current lipid-lowering therapies
| Lipid-lowering drug class | Examples | Mode of action |
| Statins (HMG-CoA reductase inhibitors) | Simvastatin, atorvastatin, rosuvastatin, pravastatin, fluvastatin | Inhibit cholesterol synthesis; ↑ LDLR |
| Cholesterol absorption inhibitors | Ezetimibe | Reduce enterohepatic cholesterol cycling, ↑ LDLR |
PCSK9 inhibitors
|
Alirocumab, evolocumab Inclisiran |
Reduce PCSK9 binding to LDLRs, ↑ LDLR Inhibits translation and hepatic production of PCSK9 |
| Bile acid sequestrants | Colesevelam, colestipol, cholestyramine | Divert cholesterol into bile acid synthesis, ↑ LDLR |
| Fibrates | Bezafibrate, ciprofibrate, fenofibrate and gemfibrozil | Induce lipoprotein lipase and other genes |
| Omega-3 fatty acids | Fish oils | Thought to ↓ plasma TGs by ↑ fatty acid oxidation, which suppresses hepatic lipogenesis and subsequent VLDL production |
| EPA ethyl ester | Icosapent ethyl | Likely multifactorial including TG-rich lipoprotein reduction, anti-inflammatory, antioxidant and antiplatelet effects |
| ATP citrate lyase inhibitors | Bempedoic acid | inhibits cholesterol synthesis, ↑ LDLR |
| Key: EPA = eicosapentaenoic acid; HMG-CoA = β-hydroxy-β-methylglutaryl-coenzyme A; LDL = low-density lipoprotein; LDLR = low-density lipoprotein receptor; PCSK9 = proprotein convertase subtilisin/kexin type 9; siRNA = small interfering ribonucleic acid; TG = triglyceride; VLDL = very-low-density lipoprotein | ||
Management of dyslipidaemias in different settings
This current module reviews specific treatments rather than specific lipid disorders. These are dealt with comprehensively in the ESC/EAS guidelines, which cover the different clinical settings where dyslipidaemia may be found.8 These include:
- familial dyslipidaemias e.g. FH
- children
- elderly
- metabolic syndrome and DM
- acute coronary syndrome/percutaneous coronary revascularisation
- heart failure
- valvular diseases
- autoimmune diseases
- renal disease
- transplantation patients
- peripheral arterial disease
- stroke
- HIV.
The way in which dyslipidaemias are treated varies with the clinical presentation, many of which require referral to secondary care. While the same drugs may be utilised, these may be used in different doses, combinations, and durations of time according to the clinical setting.
Familial hypercholesterolaemia (FH)
An example of one of the clinical presentations of dyslipidaemia requiring specialist care is FH. Plasma lipid levels are largely determined genetically and one of the most extreme forms of inherited hyperlipidaemia is FH. Table 5 shows the familial lipid disorders associated with CHD. HeFH may affect as many as one in 250 of the population (see module 3). Levels of total cholesterol and LDL-C are approximately twice the levels of normal in these patients, many of whom develop severe premature CHD. Use the Simon Broom criteria to aid diagnosis of FH. These patients should be referred to a lipid clinic for counselling, education, and family (cascade) testing in addition to optimisation of their treatment. High-intensity statins (atorvastatin, rosuvastatin) are the mainstay of treatment, but even these may be insufficient to achieve the >50% reduction from baseline required to normalise LDL-C levels. Patients may require combination treatment with ezetimibe, PCSK9 inhibitors or inclisiran (with less frequent use of bile acid sequestrants). For more information, see NICE guidance on FH.40
Table 5. Family matters – familial lipid disorders associated with CHD
| Familial lipid disorder associated with CHD | % of CHD |
| Familial combined hyperlipidaemia (FCH), including apolipoprotein B excess | 19% |
| Familial lipoprotein(a) excess (no dyslipidaemia) | 19% |
| Familial dyslipidaemia (↑ TG, ↓ HDL) | 15% |
| Familial hypoalphalipoproteinaemia (FHA) | 4% |
| Familial hypercholesterolaemia (FH) | 3% |
| Familial hypertriglyceridaemia | 1% |
| >50% of premature CHD have identifiable familial disorder Key: CHD = coronary heart disease; HDL = high-density lipoprotein; Lp(a) = lipoprotein(a); TG = triglyceride Adapted from Genest JJ et al, 199239 |
|
Lomitapide, a microsomal TG transfer protein (MTP) inhibitor, is licensed only for HoFH under specialist lipid clinic. Patients require vitamin E and fatty acid supplementation with this treatment. Regular monitoring of liver function test and annual screening for liver steatosis and fibrosis is necessary.
Lipoprotein apheresis
Lipoprotein apheresis resembles dialysis and is a technique which physically removes LDL-C from the bloodstream, typically requiring fortnightly treatment in a specialist centre lasting several hours per session.
Strategies for optimising treatment
European guidelines suggest that ‘no smoking, healthy eating and being physically active are the foundations of preventive cardiology’.8 Many patients, particularly those with inherited conditions, will not achieve cholesterol treatment targets with lifestyle interventions alone. Most patients will therefore require lipid-modifying drug treatments.
Response to lipid lowering medications may be disappointingly poor in patients with the most severe forms of FH – compound HeFH and HoFH – who may require treatment with lipoprotein apheresis.42
Box 1. Barriers to achieving treatment goals
| Poor drug adherence |
| Poor adherence to dietary changes resulting in weight gain |
| Poor response to drug therapy |
| Inadequate follow up and monitoring |
| Intolerance to drug therapy |
| Development of a secondary hyperlipidaemia |
Adherence to statin treatment is poor with up to one-third or more of patients stopping their medication within a year. This poor adherence and the reality that prescribers do not sufficiently up-titrate the dose of statin are among the reasons why over half of all CHD patients and about four out of five high-risk patients are not achieving treatment goals.8 A number of other reasons why patients fail to reach treatment goals are shown in box 1. Advice on how this may be improved involves developing a good alliance with the patient and other approaches.
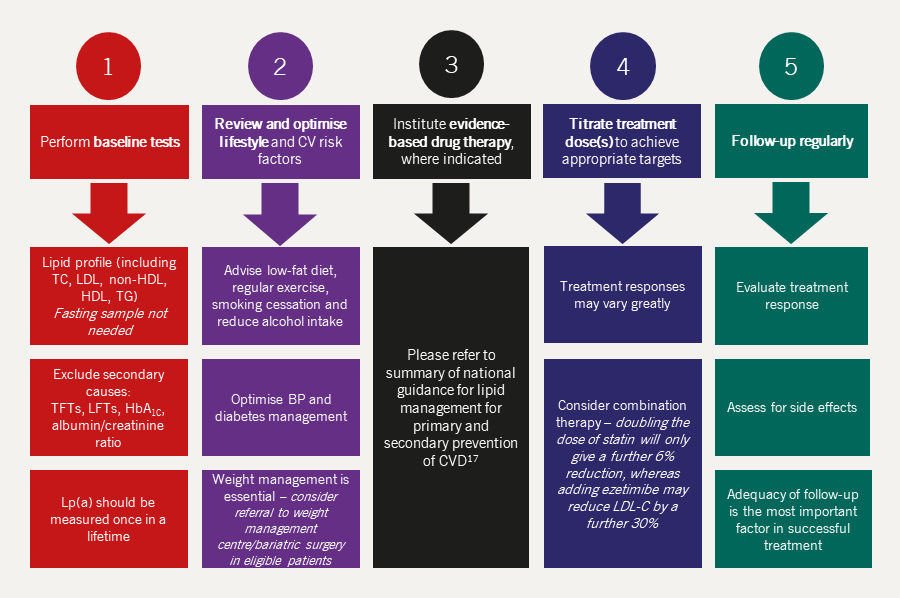
| Key: BP = blood pressure; CK = creatine kinase; CV = cardiovascular; CVD = cardiovascular disease; HbA1C = glycated haemoglobin A1C; HDL = high-density lipoprotein; LDL = low-density lipoprotein; LDL-C = low-density lipoprotein cholesterol; LFTs = liver function tests; Lp(a) = lipoprotein(a); TC = total cholesterol; TG = triglyceride |
Who should be referred to a lipid clinic?17
- Patients with suspected familial hyperlipidaemia
- Patients with progressive CVD despite maximum tolerated lipid-lowering therapy
- Patients with severe hypertriglyceridaemia at risk of pancreatitis
- Patients with serious statin intolerance
- Patients in whom there is uncertainty about diagnosis.
Future therapeutic targets and therapies
Lipoprotein(a)
Lipoprotein(a) (Lp[a]) is an independent risk marker for atherosclerosis and calcific aortic valve stenosis.8,12 Lp(a) is a composite particle which contains apolipoprotein B, in common with LDL, but it also contains a unique plasminogen-like protein, apolipoprotein(a) (apo[a]), with a greater number of structural variants (isoforms) than other apolipoproteins. The plasma level of Lp(a) is to a major extent a reflection of the hepatic production rate which is genetically determined and dependent on the isoform size. Crucially, Lp(a) is not removed by the LDLR and, unlike LDL, clearance is unaffected by statins. PCSK9 inhibitors reduce Lp(a) levels modestly,12 but currently no specific Lp(a)-lowering medication is available and the main management is to address other modifiable risk factors intensively. Weight management and addressing lifestyle factors, blood pressure, glucose and dyslipidaemia is crucial. For management of dyslipidaemia in patients with elevated Lp(a) levels >90 nmol/L, the HEART UK consensus recommend achieving a greater than 50% reduction in non-HDL-C.12 Alternatively, a non-HDL-C target of <2.5 nmol/L (LDL-C less than approximately 1.8 mmol/L) is recommended.12 Lipoprotein apheresis is the only means to achieve substantial reduction of Lp(a) and should be considered in patients with progressive CHD and a history of recurrent CV events despite optimal lipid-lowering treatment and elevated Lp(a)(more than approximately 150 nmol/L); however, its use is restricted due to its cost and limited access.12
For primary prevention, aspirin might be considered on a case-by-case basis. Although it is not routinely recommended in the primary prevention of patients with elevated Lp(a), the Women’s Health Study showed that patients with elevated Lp(a) benefit from aspirin.43 Results of more recent randomised controlled trials also demonstrated that aspirin reduced the incidence of MACE in elderly carriers of elevated Lp(a)-associated genotype without significantly increasing bleeding risk.44 Based on these studies, aspirin might be considered in patients with significant CVD risk and elevated Lp(a) levels on a case-by-case basis.
To date, there has been no clinical trial evidence to show that reduction in Lp(a) leads to improvement in CV outcomes, although evidence from genetic Mendelian randomisation studies predict it to be a causative risk factor. A number of new antisense oligonucleotide- and siRNA-based therapies for Lp(a) lowering are currently undergoing clinical trials.
Lp(a) is not currently recommended for risk screening in the general population, but Lp(a) measurement once in one’s lifetime should be considered in people with a high risk of CVD and in patients with FH or a strong family history of premature atherothrombotic disease.12
An Lp(a) risk calculator is a new useful tool for estimating CVD risk in patients with elevated Lp(a). This assessment tool highlights the importance of Lp(a) in risk assessment and how CVD risk is significantly underestimated when elevated Lp(a) is not considered. Although, at present, there is no treatment available for lowering Lp(a), this tool guides clinicians and motivates patients to manage other modifiable risk factors more rigorously. The Lp(a) risk calculator is available at: https://www.lpaclinicalguidance.com/
HDL-C as a treatment target
There is conclusive evidence that lowering LDL-C levels with statins reduces the risk of CVD events. Statins, even when used optimally, do not always afford complete vascular protection and substantial residual CV risk persists, despite best treatment efforts. Some of this ‘residual risk’ will be determined by modifiable risk factors, such as lipids, hypertension, smoking and DM. Alternative approaches to reduce this risk include further reductions in apolipoprotein (apo) B-containing atherogenic lipoproteins or increasing atheroprotective lipoproteins, specifically HDL-C.
In contrast to total cholesterol, LDL-C and TGs, HDL-C is inversely correlated with CVD. Low HDL-C levels (<1 mmol/L in men and <1.2 mmol/L in women) are associated with increased CHD risk, whereas HDL-C levels of up to 2–2.5 mmol/L are protective against atherosclerosis. This may be because of reverse cholesterol transport (see module 1), but HDL-C has numerous functions independent of lipid metabolism (figure 11), including anti-inflammatory activity, which may be cardioprotective. Conversely, it has been reported that significantly raised HDL-C (>2.5 mmol/L) is associated with atherosclerosis and CVD. Therefore, the TC/HDL-C ratio (which is used in QRISK®3) may underestimate CVD risk in patients with significantly elevated HDL-C.
Mechanisms of the atheroprotective effects of HDL-C
HDL-C is postulated to protect against CVD by mediating reverse cholesterol transport, a protective mechanism against atherosclerosis. Additionally, HDL-C has other atheroprotective effects, namely that of antioxidant, antifibrotic, antiapoptotic, anti-inflammatory and direct beneficial endothelial effects.44 Each of these mechanisms are further expanded upon in figures 11–15 (adapted from Hausenloy and Yellon, 200845).
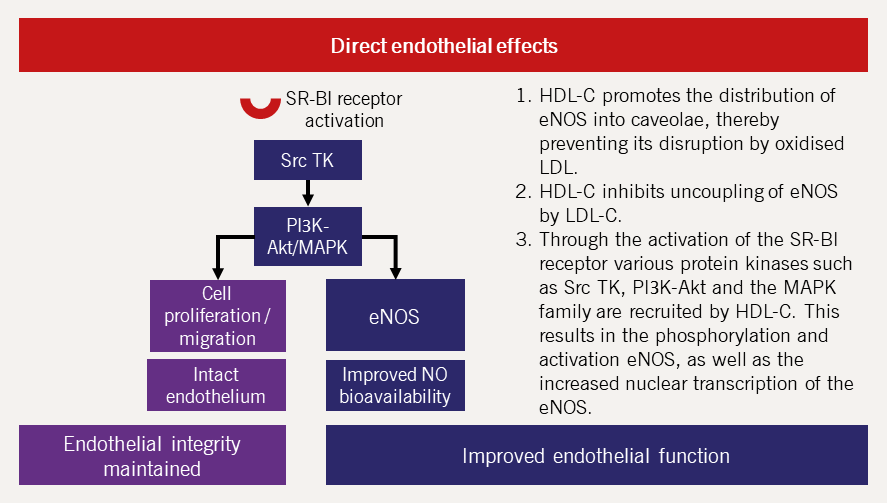
| Key: eNOS = endothelial nitric oxide synthase; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; MAPK = mitogen-activated protein kinases; PIЗK-Akt = phosphoinositide-З-kinase–protein kinase B; SR-BI = scavenger receptor class B type I; Src TK = Src tyrosine kinase |
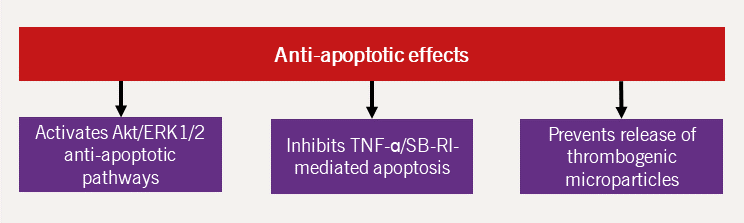
| Key: Akt = protein kinase B; ERK1/2 = extracellular signal-regulated kinase 1/2; SB-RI = scavenger receptor class B type I; TNF = tumour necrosis factor |
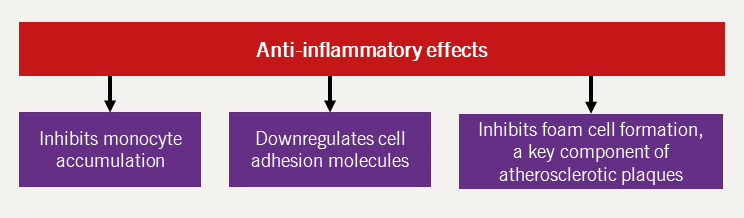
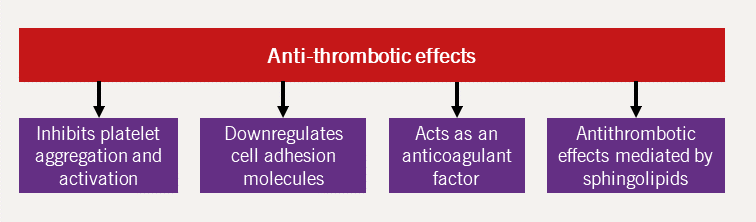
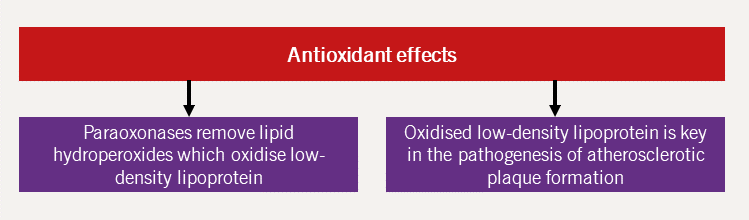
Increasing HDL-C levels
Whilst low HDL-C is associated with CVD risk, several randomised trials failed to show any improvement in major cardiac event rate by increasing HDL-C.
Evidence that raising HDL-C prevents CV events is still being tested in clinical trials. There are relatively few options available for raising HDL-C. Lifestyle measures, such as weight reduction, vigorous exercise, smoking cessation, may improve HDL-C levels. Increased alcohol intake can increase HDL-C. The therapeutic class of cholesteryl ester transfer protein (CETP) inhibitors can raise HDL-C levels substantially by approximately 30%, but investigations with the first CETP inhibitor, torcetrapib, were terminated due to excess mortality and major CV events, which were associated with increases in blood pressure. The CETP inhibitors dalcetrapib and anacetrapib were also withdrawn due to the lack of a significant beneficial effect on clinical outcomes.
Key messages
- Effective treatment is available for most hyperlipidaemias
- Well-tolerated and powerful LDL-lowering drug therapies (statins, ezetimibe, PCSK9 inhibitors, inclisiran and bempedoic acid), can be given alone or in combination to upregulate the LDLR pathway, and have replaced older drugs (such as resins and niacin)
- For every 1 mmol/L reduction in LDL-C level, there is a corresponding reduction in the risk of developing coronary heart disease by approximately 20% over five years
- Combination treatments have an additive benefit commensurate with the achieved reduction of non-HDL-C levels
- Fibrates are of particular value in the management of severe hypertriglyceridaemia, which persists despite dietary and lifestyle measures and when secondary causes have been addressed
- Icosapent ethyl has CV benefits in patients on statins with high CVD risk and moderately raised TG levels.
Recommended reading
- Nicholls P, Young I (eds). Lipid disorders. Oxford Cardiology Library. Oxford: Oxford University Press, 2009. ISBN 978-0-19-956965-6.
close window and return to take test
References
- Robinson JG, Wang S, Smith BJ, Jacobsen TA. Meta-analysis of the relationship between non-high-density lipoprotein cholesterol reduction and coronary heart disease risk. J Am Coll Cardiol 2009;53:316–22. https://doi.org/10.1016/j.jacc.2008.10.024
- Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation 2007;116:1832–44. https://doi.org/10.1161/CIRCULATIONAHA.106.676890
- Ference BA, Yoo W, Alesh I et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J Am Coll Cardiol 2012;60:2631–9. http://doi.org/10.1056/NEJMoa054013
- Cohen JC, Boerwinkle E, Mosley TH, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006;354:1264–72. https://doi.org/10.46747/cfp.6910701
- Latimer J, Batty JA, Neely RD, Kunadian V. PCSK9 inhibitors in the prevention of cardiovascular disease. J Thrombos Thrombolysis 2016;42:405–19. https://doi.org/0.1007/s11239-016-1364-1
- Baigent C, Blackwell L, Emberson J et al; Cholesterol Treatment Trialists Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81. https://doi.org/10.1016/S0140-6736(10)61350-5
- National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification [NG238]. London: NICE. Published December 2023. Available at: https://www.nice.org.uk/guidance/ng238 (accessed April 2024)
- Mach F, Baigent C, Catapano AL et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–88. https://doi.org/10.1093/eurheartj/ehz455
- Marx N, Federici M, Schütt K et al; ESC Scientific Document Group. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J 2023;44:4043–140. https://doi.org/10.1093/eurheartj/ehad192
- Byrne R, Rossello X, Coughlan J et al. 2023 European Society of Cardiology Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J 2023;44:3720–826. https://doi.org/10.1093/eurheartj/ehad191
- NHS England. Quality and outcomes framework guidance for 2023/2024. NHS England, 2023. Available at: https://www.england.nhs.uk/gp/investment/gp-contract/quality-on-outcomes-framework-qof-changes (accessed April 2024)
- Cegla J, Neely RDG, France M et al. HEART UK consensus statement on lipoprotein(a): a call to action. Atherosclerosis 2019;291:62–70. https://doi.org/10.1016/j.atherosclerosis.2019.10.011
- National Institute for Health and Care Excellence. Obesity: identification, assessment and management [CG189]. London: NICE, 2014, updated July 2023. Available at: https://www.nice.org.uk/guidance/cg189 (accessed April 2024)
- Trautwein EA, Vermeer MA, Hiemstra H, Ras RT. LDL-cholesterol lowering of plant sterols and stanols-which factors influence their efficacy? Nutrients 2018;10:1262. https://doi.org/10.3390/nu10091262
- Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr 1999;69:30–42. https://doi.org/10.1093/ajcn/69.1.30
- Viljoen A. Improving dyslipidaemia management: focus on lifestyle intervention and adherence issues. Br J Cardiol 2012;19(suppl 1):S9–S11. https://doi.org/10.5837/bjc.2012.s03
- NHS Accelerated Access Collaborative. Summary of national guidance for lipid management for primary and secondary prevention of CVD. Last updated January 2024. Available at: https://www.england.nhs.uk/aac/wp-content/uploads/sites/50/2020/04/lipid-management-pathway-v6.pdf (accessed March 2024)
- National Institute for Health and Care Excellence. Statins could be a choice for more people to reduce their risk of heart attacks and strokes, says NICE. 2023. Available at: https://www.nice.org.uk/news/article/statins-could-be-a-choice-for-more-people-to-reduce-their-risk-of-heart-attacks-and-strokes-says-nice (accessed March 2024)
- Hussaini SH, Farrington EA. Idiosyncratic drug-induced liver injury: an overview. Expert Opin Drug Saf 2007;6:673–84. https://doi.org/10.1517/14740338.6.6.673
- Ray K, Raal F, Kallend D et al; ORION Phase III investigators. Inclisiran and cardiovascular events: a patient-level analysis of phase III trials. Eur Heart J 2023;44:129–38. https://doi.org/10.1093/eurheartj/ehac594
- Cannon CP, Blazing MA, Giugliano RP et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–97. https://doi.org/10.1056/NEJMoa1410489
- National Institute for Health and Care Excellence. Ezetimibe for treating primary heterozygous-familial and non-familial hypercholesterolaemia [TA385]. London: NICE. Published February 2016. Available at: https://www.nice.org.uk/guidance/ta385 (accessed April 2024)
- Räber L, Ueki Y, Otsuka T et al. Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction: The PACMAN-AMI randomized clinical trial. JAMA 2022;327:1771–81. https://doi.org/10.1001/jama.2022.5218
- Nicholls S, Kataoka Y, Nissen S et al. Effect of evolocumab on coronary plaque phenotype and burden in statin-treated patients following myocardial infarction. J Am Coll Cardiol Imaging 2022;15:1308–21. https://doi.org/10.1016/j.jcmg.2022.03.002
- National Institute for Health and Care Excellence. Alirocumab for treating primary hypercholesterolaemia and mixed dyslipidaemia [TA393] London: NICE. Published June 2016. Available at: https://www.nice.org.uk/guidance/ta393 (accessed April 2024)
- National Institute for Health and Care Excellence. Evolocumab for treating primary hypercholesterolaemia and mixed dyslipidaemia [TA394]. London: NICE. Published June 2016. Available at: https://www.nice.org.uk/guidance/ta394 (accessed April 2024)
- National Institute for Health and Clinical Excellence. Inclisiran for treating primary hypercholesterolaemia or mixed dyslipidaemia [TA733]. London: NICE, October 2021 Available at: https://www.nice.org.uk/guidance/ta733 (accessed April 2024)
- Chi X, Gatti P, Papoian T. Safety of antisense oligonucleotide and siRNA-based therapeutics. Drug Discovery Today 2017;5:823–33. https://doi.org/10.1016/j.drudis.2017.01.013
- Wright RS, Raal FJ, Koenig W et al. ORION-8: Long-term efficacy and safety of twice-yearly inclisiran in high cardiovascular risk patients. Data presented at the European Society of Cardiology Congress August 28th 2023, Amsterdam, The Netherlands. Available at: https://www.novartis.com/news/media-releases/novartis-presents-new-long-term-leqvio-inclisiran-data-demonstrating-consistent-efficacy-and-safety-beyond-six-years (accessed April 2024)
- Ray K, Raal F, Kallend D et al; ORION Phase III investigators. Inclisiran and cardiovascular events: a patient-level analysis of phase III trials. Eur Heart J 2023;44:129–38. https://doi.org/10.1093/eurheartj/ehac594
- Wright RS, Ray KK, Raal FJ et al. Pooled patient-level analysis of inclisiran trials in patients with familial hypercholesterolemia or atherosclerosis. J Am Coll Cardiol 2021;77:1182–93. https://doi.org/10.1016/j.jacc.2020.12.058
- Landray MJ, Haynes R, Hopewell JC et al; HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–12. https://doi.org/10.1056/NEJMoa1300955
- Bornfeldt KE. Triglyceride lowering by omega-3 fatty acids: a mechanism mediated by N-acyl taurines. J Clin Invest 2021;131:e147558. https://doi.org/10.1172/JCI147558
- National Institute for Health and Clinical Excellence. Icosapent ethyl with statin therapy for reducing the risk of cardiovascular events in people with raised triglycerides [TA805]. London: NICE, July 2022. Available at: https://www.nice.org.uk/guidance/ta805 (accessed April 2024)
- Bhatt DL, Steg PG, Miller M et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. https://doi.org/10.1056/NEJMoa1812792
- Vazkepa® (icosapent ethyl) Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/12964/smpc#gref (accessed May 2024)
- Prescribing information Nexletol™ (bempedoic acid). Available from Esperion Therapeutics, Inc.,2020.
- National Institute for Health and Clinical Excellence. Bempedoic acid with ezetimibe for treating hypercholesterolaemia or mixed dyslipidaemia [TA694] London: NICE. Published April 2021. Available at: https://www.nice.org.uk/guidance/ta694 (accessed April 2024)
- Nissen SE, Lincoff AM, Brennan D et al; CLEAR Outcomes Investigators. Bempedoic acid and cardiovascular outcomes in statin-Intolerant patients. N Engl J Med 2023;388:1353–64. https://doi.org/10.1056/NEJMoa2215024
- National Institute for Health and Care Excellence. Identification and management of familial hypercholesterolaemia [CG71]. London: NICE. Published August 2008, last updated October 2019. Available at: http://nice.org.uk/guidance/CG71/ (accessed April 2024)
- Genest JJ Jr, Martin-Munley SS, McNamara JR et al. Familial lipoprotein disorders in patients with premature coronary artery disease. Circulation 1992;85:2025–33. http://doi.org/10.1161/01.CIR.85.6.2025
- Varghese MJ. Familial hypercholesterolemia: a review. Ann Pediatr Cardiol 2014;7:107–17. https://doi.org/10.4103/0974-2069.132478
- Chasman DI, Shiffman D, Zee RYL et al. Polymorphism in the apolipoprotein(a) gene, plasma lipoprotein(a), cardiovascular disease, and low-dose aspirin therapy. Atherosclerosis 2009;203:371–6. https://doi.org/10.1016/j.atherosclerosis.2008.07.019
- Lacaze P, Bakshi A, Riaz M et al. Aspirin for primary prevention of cardiovascular events in relation to lipoprotein(a) genotypes. J Am Coll Cardiol 2022;80:1287–98. https://doi.org/10.1016/j.jacc.2022.07.027
- Hausenloy DJ, Yellon DM. Targeting residual cardiovascular risk: raising high-density lipoprotein cholesterol. Heart 2008;94:706–14. http://doi.org/10.1136/hrt.2007.125401

All rights reserved. No part of this programme may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior permission of the publishers, Medinews (Cardiology) Limited.
It shall not, by way of trade or otherwise, be lent, re-sold, hired or otherwise circulated without the publisher’s prior consent.
Medical knowledge is constantly changing. As new information becomes available, changes in treatment, procedures, equipment and the use of drugs becomes necessary. The editors/authors/contributors and the publishers have taken care to ensure that the information given in this text is accurate and up to date. Readers are strongly advised to confirm that the information, especially with regard to drug usage, complies with the latest legislation and standards of practice.
Healthcare professionals should consult up-to-date Prescribing Information and the full Summary of Product Characteristics available from the manufacturers before prescribing any product. Medinews (Cardiology) Limited cannot accept responsibility for any errors in prescribing which may occur.