Only a small proportion of patients requiring pacemaker or defibrillator implantation have congenital cardiac abnormalities. Patients with such anomalies can be divided into two categories: those with undiscovered congenital abnormalities, which had not given rise to symptoms or other obvious physical signs, and those known to have congenital abnormalities having had surgical intervention or not.
Pacemaker implantation in these two groups of patients may give rise to practical challenges and the implanting physician should be familiar with them so that potential problems can readily be recognised. In this, and the subsequent articles, we will cover the most common congenital cardiac anomalies with relevance to cardiac device implantation.
Introduction
The vast majority of patients requiring pacemaker or defibrillator implantation have structurally normal hearts and patients with congenital cardiac abnormalities constitute only a small proportion. The latter can be divided into two groups. The first includes those with undiscovered congenital abnormalities, which do not give rise to symptoms or obvious physical signs, such as dextrocardia, persistent left-sided superior vena cava, atrial septal defect and patent foramen ovale. The second group includes those who are known to have structural cardiac abnormalities, such as Ebstein’s anomaly, ventricular septal defect, transposition of great arteries, tetralogy of Fallot and tricuspid atresia, and some patients will have already undergone corrective cardiac surgery. These patients require special consideration before proceeding to the pacing theatre. In particular, the operator will need to know whether a transvenous approach is feasible, what problems might be encountered during lead implantation and how to seek the best and most stable of lead positions.
Indications for device implantation
Many congenital cardiac defects are accompanied by abnormalities of the conduction system, either directly related to developmental abnormalities or as a consequence of the unique myocardial substrate created by large septal patches, extensive suture lines, long-standing cyanosis or abnormal pressure/volume status.1 Abnormalities vary from sick sinus syndrome, sino-atrial block, sinus arrest to atrio-ventricular (AV) disturbances, such as second- or third-degree AV block. Symptoms may include dizziness, syncope, heart failure or, simply, effort intolerance, lassitude or fatigue. Complete AV block carries a prognostic disadvantage and such patients require pacing whether they are symptomatic or not. Moreover, ventricular tachyarrhythmias may occur in patients with severe and complex congenital heart disease, particularly in those with complex cyanotic heart disease, Eisenmenger’s syndrome, after corrective surgical procedures, and in those with impaired left ventricular function. Such patients may require an implantable cardiac defibrillator (ICD), which may present a challenge to the implanting cardiologist.
Investigations prior to device implantation
All patients being put forward for elective device implantation require pre-operative investigations. These should include a 12-lead electrocardiogram (ECG) and a chest X-ray. These simple investigations may be diagnostic in some cases, such as dextrocardia, or may help to raise suspicion of congenital heart disease. Further investigations may include transthoracic echocardiography, transoesophageal echocardiography, computed tomography (CT), magnetic resonance imaging (MRI) and cardiac catheterisation. Where appropriate, the main aim of these tests is to clarify the cardiac anatomy in order to safely embark on transvenous lead placements. For those patients with known congenital cardiac defects, reviewing previous surgical records (following cardiac surgery in earlier life), or previous cardiac catheterisation information, may be useful in identifying anatomical abnormalities. In recent times, with the advent of biventricular pacing, it is relevant also to know the position of the coronary sinus, for example in patients with Ebstein anomaly who have already undergone tricuspid valve replacement.
It goes without saying that many young patients who receive permanent pacemakers as children following surgery for their congenital heart disease, will require several further procedures in the years to come. These range from generator change (at end of battery life), lead problems requiring replacement of a lead, to system revisions for infection and intervention for venous thrombosis/occlusion.
In this article, we will discuss those anomalies that are usually encountered by chance, at or just prior to, implantation: patent foramen ovale/atrial septal defect, Ebstein’s anomaly and ventricular septal defect. In the subsequent two papers, the challenge of device implantation in patients with more complex and usually recognised congenital structural cardiac defects will be discussed.
Dextrocardia
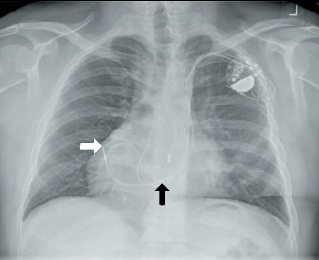
The prevalence of situs inversus varies among different populations, but is less than one in 10,000 people. This positional abnormality, in which the cardiac apex is located on the right side of the chest, should not pose a problem to pacemaker implantation once the cardiologist is oriented, as long as no other cardiac structural defects are associated with the dextrocardia. Provided that the pre-paced rhythm is not complete heart block with a wide QRS complex, the 12-lead ECG should give the diagnosis before the patient enters the pacing theatre! If not, fluoroscopy will suggest the abnormality (figure 1). Some current fluoroscopy software allow live left–right inversion of the image, which can be helpful for the implanting physician as it provides a projection that looks more familiar.
Persistent left-sided superior vena cava
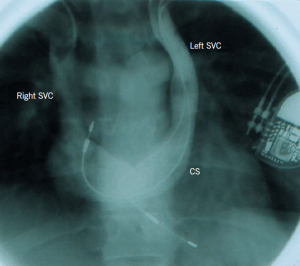
This is the most common congenital venous anomaly in the chest. Although it is prevalent only in 0.3–0.5% of the general population, persistent left-sided superior vena cava (SVC) may be present in 4.3–11% of patients with congenital heart disease.2 The anomaly is due to failure of regression of the left anterior and common cardinal veins and left sinus horn. The persistent left SVC starts at the junction of the left subclavian vein and left internal jugular vein, passes lateral to the aortic arch and receives the left superior intercostal vein. It then passes anterior to the left hilum, is joined by the hemiazygous system, crosses the posterior wall of the left atrium and receives the great cardiac vein to become the coronary sinus.
In 92% of cases, the left-sided SVC enters the coronary sinus (CS) (figure 2a). In this configuration, there is little if any functional impact as blood from the head and arms still reaches the right atrium (RA). In the remaining 8% of cases, drainage is into the left atrium (LA), in which case the patient usually has a small right to left shunt.
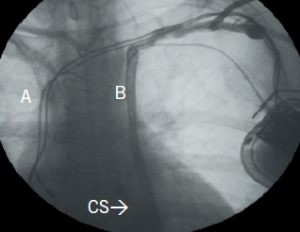
In 65–75% of cases, the left brachiocephalic vein is absent or small and, therefore, there is no bridging connection to the right SVC (figure 2a). The majority (90%) of patients will have bilateral SVC, which is also termed SVC duplication (figures 2a and 2b).
To be able to achieve left-sided pacemaker implantation, the physician usually tries to manoeuvre the atrial and ventricular leads into ideal positions in the RA and right ventricle (RV), respectively, as they course through the coronary sinus. Although this can be challenging, persistence and use of active fixation leads usually proves successful (figure 2c). Thrombosis of a large coronary sinus following pacemaker lead implantation has been reported. Left ventricular pacing via a cardiac venous tributary is usually not difficult to achieve, but persistence in placing a lead into the RV is often worthwhile in pacemaker-dependent patients. Another way to achieve left-sided implantation is by passing the lead through the bridging venous connection (present in 25–35%) to the right SVC (figure 2b). If left-sided implantation is not feasible, then a right-sided venogram should be performed to ascertain the presence of a right SVC. Finally, if all previous attempts are exhausted, trans-IVC (inferior vena cava) or epicardial lead implant can be considered as a last resort.
Atrial septal defect/persistent foramen ovale
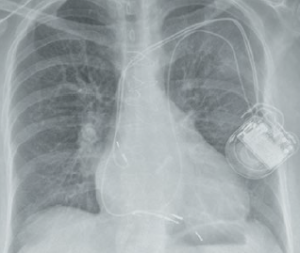
Generally, isolated foramen ovale and atrial septal defect do not hinder permanent pacemaker implantation. However, it is possible for the lead to enter the LA and then left ventricle (LV), and if positioned there may inadvertently result in systemic embolisation – including stroke. They should be removed or the patient anticoagulated. The lateral chest X-ray will suggest a posterior position of the lead indicating left ventricular placement. The lead will arch over the atrial septum and the ECG will confirm LV pacing.
When an atrial septal defect has been repaired by a patch or a closure device, atrial septal pacing may not be possible because of fibrosis. Moreover, if the right-sided chambers are dilated as a result of a long-standing defect, active fixation leads should be preferred in order to prevent lead displacement.
Ebstein’s anomaly
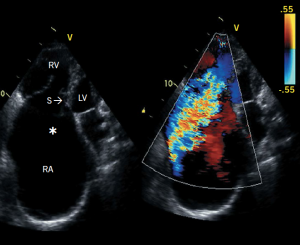
Ebstein’s anomaly is characterised by failure of delamination of the tricuspid valve leaflets with resultant apical displacement of the septal leaflet (± posterior leaflet) (figure 3). The displaced tricuspid valve divides the RV into two parts – an atrialised portion lying between the tricuspid annulus and the displaced tricuspid orifice, and the remainder of the true RV, which lies beyond the tricuspid valve.
The relevance to pacemaker implanters is that approximately 20–25% of such patients develop arrhythmias and conduction abnormalities and 3–4% require pacing.3 The most common indications for pacing include persistent atrial standstill and AV block (de novo, post-AV node ablation or post-surgery). The atrialised portion of the RV varies in size, muscularity and thickness, but it has the electrophysiological characteristics of the RV. Hence, the RV lead can be placed above the valve rather than through it. Active fixation leads should be used in the atrium, the atrialised portion of the RV and in the RV apex or RV outflow tract to avoid displacement in such patients, who often have significant tricuspid regurgitation. It is worth remembering that other congenital cardiac defects may co-exist with Ebstein’s anomaly, e.g. atrial septal defect, ventricular septal defect. When it is impossible to insert electrodes in the right atrium and ventricle, one may use the cardiac veins via the coronary sinus to achieve left ventricular pacing. However, in pacemaker-dependent patients, epicardial pacing may be more appropriate.
During the surgical correction for the tricuspid valve (reconstruction/replacement), a pacing lead can be inserted intra-operatively. After annuloplasty, the lead can be placed across the valve in the usual fashion. If valve replacement is required, a lead can be buried behind the sewing ring and the lead tunnelled to the anterior abdominal wall or pectoral region and connected to a generator, or capped for future use. Generally, however, if a mechanical prosthesis is implanted, epicardial pacing should be the method of choice, although, even here, endocardial pacing is not impossible if the coronary sinus is positioned on the atrial side of the prosthesis. Permanent pacing leads can be placed across a bio-prosthetic tricuspid valve without too much difficulty.
Ventricular septal defect
Patients with small ventricular septal defects that do not warrant closure may require pacemaker implantation. Care should be taken to be sure that the lead does not cross into the LV where systemic embolisation and stroke may be an associated risk.
Patients who have had surgical or device closure of a ventricular septal defect may be difficult to pace endocardially due to scarring/fibrosis or synthetic material at the RV apex or within the interventricular septum. A regurgitant tricuspid valve and enlarged/scarred right atrium/ventricle can result in difficulty placing leads into stable, effective positions, and, generally, active fixation leads should be used from the outset.
Conflict of interest
None declared.
References
- Franco D, Icardo JM. Molecular characterization of the ventricular conduction system in the developing mouse heart: topographical correlation in normal and congenitally malformed hearts. Cardiovasc Res 2001;49:417–29. http://dx.doi.org/10.1016/S0008-6363(00)00252-2
- Sarodia BD, Stoller JK. Persistent left superior vena cava: case report and literature review. Respir Care 2000;45:411–16.
- Celermajer DS, Bull C, Till JA et al. Ebstein’s anomaly: presentation and outcome from fetus to adult. J Am Coll Cardiol 1994;23:170–6. http://dx.doi.org/10.1016/0735-1097(94)90516-9
